Alunbrig One-Month Initiation Pack TM
Listen to the Pronunciation:
You should seek medical advice in relation to medicines and use only as directed by a healthcare professional. Always read the label. If symptoms persist see your healthcare professional.
Active ingredients: brigatinib
Pack: Alunbrig One-Month Initiation Pack (7 x 90 mg tablets, 21 x 180 mg tablets), 1 pack
Brand name
(ARTG)
: ALUNBRIG brigatinib 90 mg and 180 mg film-coated tablets blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
Listen to the audio transcription of the CMI leaflet.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
ALUNBRIG is indicated for the treatment of adult patients with anaplastic lymphoma kinase (ALK)-positive locally advanced or metastatic non-small cell lung cancer (NSCLC).
How to use this medicine
(ARTG)
This medicine is a pack that contains more than one component.
Component :
- Tablet, film coated
- Oral
- Oval, white to off-white with debossed "U7" on one side and plain on the other side
Component :
- Tablet, film coated
- Oral
- Oval, white to off-white with debossed "u13" on one side and plain on the other side.
Images


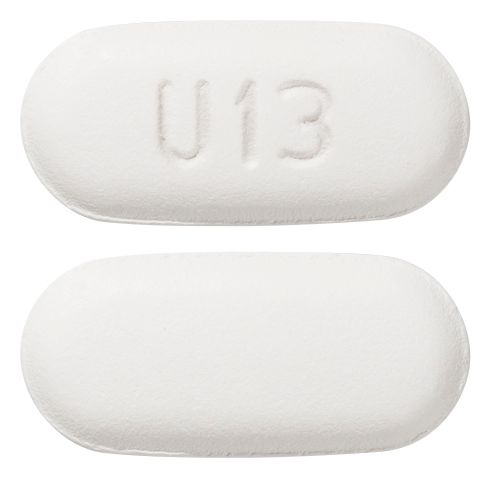

Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 30 degrees Celsius
- Shelf lifetime is 36 Months.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is
- 7 x 90 mg tablets and 21 x 180 mg tablets pack
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient brigatinib
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
This medicine is under additional monitoring as it is new or being used in a different way. You can help identify new safety information by reporting any side effects you may get.
- You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems.
- For more information on the Black Triangle Scheme and how to report side effects, see www.tga.gov.au/black-triangle-scheme









