Metformin (APX) TM
You should seek medical advice in relation to medicines and use only as directed by a healthcare professional. Always read the label. If symptoms persist see your healthcare professional.
Active ingredients: metformin
Brand name
(ARTG)
: TIH-METFORMIN metformin hydrochloride 850 mg tablet blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Metformin is indicated in the treatment of type 2 diabetes mellitus not satisfactorily controlled by diet, where the risk of lactic acidosis is minimised by excluding predisposing factors, especially impaired renal, hepatic or cardiovascular function. Metformin may be used as initial therapy or in sulfonylurea failure, either alone or in combination with a sulfonylurea or as adjuvant therapy in insulin-dependent diabetes.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, film coated
- Oral
- Round, white tablet (approx. 13mm), emobossed with MO on one side and the Arrow symbol ">" on the other.
Images



Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 30 degrees Celsius
- Shelf lifetime is 3 Years.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 10 tablets pack
- 60 tablets pack
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient metformin
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: TIH-METFORMIN metformin hydrochloride 850 mg tablet blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Metformin is indicated in the treatment of type 2 diabetes mellitus not satisfactorily controlled by diet, where the risk of lactic acidosis is minimised by excluding predisposing factors, especially impaired renal, hepatic or cardiovascular function. Metformin may be used as initial therapy or in sulfonylurea failure, either alone or in combination with a sulfonylurea or as adjuvant therapy in insulin-dependent diabetes.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, film coated
- Oral
- Round, white tablet (approx. 13mm), emobossed with MO on one side and the Arrow symbol ">" on the other.
Images



Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 30 degrees Celsius
- Shelf lifetime is 3 Years.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 10 tablets pack
- 60 tablets pack
Is this medicine subsidised ?
(PBS)
This medicine was verified as being available on the PBS (Pharmaceutical Benefits Scheme) on January, 6 2026. To learn more about this subsidy, visit the Pharmaceutical Benefits Scheme (PBS) website.
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient metformin
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: TIH-METFORMIN metformin hydrochloride 500 mg tablet blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Metformin is indicated in the treatment of type 2 diabetes mellitus not satisfactorily controlled by diet, where the risk of lactic acidosis is minimised by excluding predisposing factors, especially impaired renal, hepatic or cardiovascular function. Metformin may be used as initial therapy or in sulfonylurea failure, either alone or in combination with a sulfonylurea or as adjuvant therapy in insulin-dependent diabetes.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, film coated
- Oral
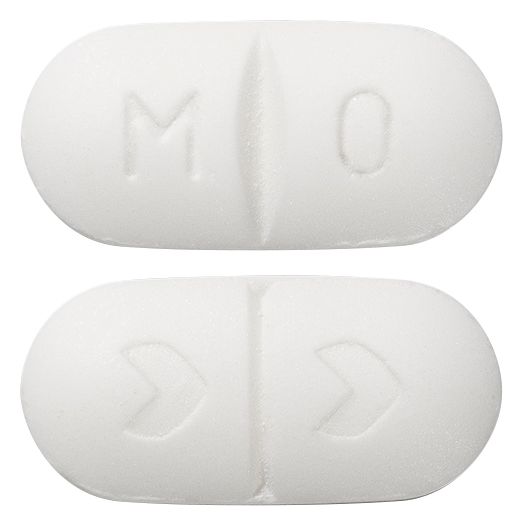
- Oblong, white tablet, embossed with M/O on one side and the Arrow symbol ">" on the other.
Images
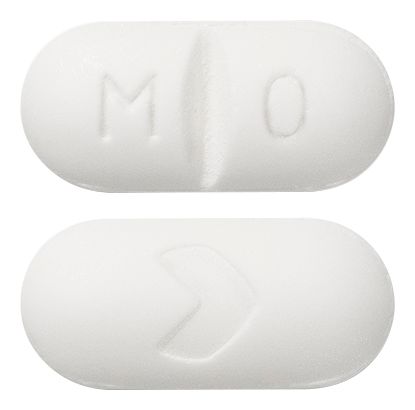

Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 30 degrees Celsius
- Shelf lifetime is 3 Years.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 10 tablets pack
- 100 tablets pack
- 20 tablets pack
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient metformin
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: TIH-METFORMIN metformin hydrochloride 1000 mg tablet blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Metformin is indicated in the treatment of type 2 diabetes mellitus not satisfactorily controlled by diet, where the risk of lactic acidosis is minimised by excluding predisposing factors, especially impaired renal, hepatic or cardiovascular function. Metformin may be used as initial therapy or in sulfonylurea failure, either alone or in combination with a sulfonylurea or as adjuvant therapy in insulin-dependent diabetes.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, film coated
- Oral
- Oval, white tablet, embossed with M/O on one side and ">/>" on the other.
Images

Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 30 degrees Celsius
- Shelf lifetime is 3 Years.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 10 tablets pack
- 100 tablets pack
- 15 tablets pack
- 60 tablets pack
- 90 tablets pack
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient metformin
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: TIH-METFORMIN metformin hydrochloride 500 mg tablet blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Metformin is indicated in the treatment of type 2 diabetes mellitus not satisfactorily controlled by diet, where the risk of lactic acidosis is minimised by excluding predisposing factors, especially impaired renal, hepatic or cardiovascular function. Metformin may be used as initial therapy or in sulfonylurea failure, either alone or in combination with a sulfonylurea or as adjuvant therapy in insulin-dependent diabetes.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, film coated
- Oral
- Oblong, white tablet, embossed with M/O on one side and the Arrow symbol ">" on the other.
Images


Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 30 degrees Celsius
- Shelf lifetime is 3 Years.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 10 tablets pack
- 100 tablets pack
- 20 tablets pack
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient metformin
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: TIH-METFORMIN metformin hydrochloride 500 mg tablet blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Metformin is indicated in the treatment of type 2 diabetes mellitus not satisfactorily controlled by diet, where the risk of lactic acidosis is minimised by excluding predisposing factors, especially impaired renal, hepatic or cardiovascular function. Metformin may be used as initial therapy or in sulfonylurea failure, either alone or in combination with a sulfonylurea or as adjuvant therapy in insulin-dependent diabetes.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, film coated
- Oral
- Oblong, white tablet, embossed with M/O on one side and the Arrow symbol ">" on the other.
Images


Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 30 degrees Celsius
- Shelf lifetime is 3 Years.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 10 tablets pack
- 100 tablets pack
- 20 tablets pack
Is this medicine subsidised ?
(PBS)
This medicine was verified as being available on the PBS (Pharmaceutical Benefits Scheme) on January, 6 2026. To learn more about this subsidy, visit the Pharmaceutical Benefits Scheme (PBS) website.
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient metformin
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: TIH-METFORMIN metformin hydrochloride 1000 mg tablet blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Metformin is indicated in the treatment of type 2 diabetes mellitus not satisfactorily controlled by diet, where the risk of lactic acidosis is minimised by excluding predisposing factors, especially impaired renal, hepatic or cardiovascular function. Metformin may be used as initial therapy or in sulfonylurea failure, either alone or in combination with a sulfonylurea or as adjuvant therapy in insulin-dependent diabetes.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, film coated
- Oral
- Oval, white tablet, embossed with M/O on one side and ">/>" on the other.
Images


Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 30 degrees Celsius
- Shelf lifetime is 3 Years.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 10 tablets pack
- 100 tablets pack
- 15 tablets pack
- 60 tablets pack
- 90 tablets pack
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient metformin
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: TIH-METFORMIN metformin hydrochloride 1000 mg tablet blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Metformin is indicated in the treatment of type 2 diabetes mellitus not satisfactorily controlled by diet, where the risk of lactic acidosis is minimised by excluding predisposing factors, especially impaired renal, hepatic or cardiovascular function. Metformin may be used as initial therapy or in sulfonylurea failure, either alone or in combination with a sulfonylurea or as adjuvant therapy in insulin-dependent diabetes.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, film coated
- Oral
- Oval, white tablet, embossed with M/O on one side and ">/>" on the other.
Images


Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 30 degrees Celsius
- Shelf lifetime is 3 Years.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 10 tablets pack
- 100 tablets pack
- 15 tablets pack
- 60 tablets pack
- 90 tablets pack
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient metformin
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: TIH-METFORMIN metformin hydrochloride 1000 mg tablet blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Metformin is indicated in the treatment of type 2 diabetes mellitus not satisfactorily controlled by diet, where the risk of lactic acidosis is minimised by excluding predisposing factors, especially impaired renal, hepatic or cardiovascular function. Metformin may be used as initial therapy or in sulfonylurea failure, either alone or in combination with a sulfonylurea or as adjuvant therapy in insulin-dependent diabetes.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, film coated
- Oral
- Oval, white tablet, embossed with M/O on one side and ">/>" on the other.
Images


Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 30 degrees Celsius
- Shelf lifetime is 3 Years.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 10 tablets pack
- 100 tablets pack
- 15 tablets pack
- 60 tablets pack
- 90 tablets pack
Is this medicine subsidised ?
(PBS)
This medicine was verified as being available on the PBS (Pharmaceutical Benefits Scheme) on January, 6 2026. To learn more about this subsidy, visit the Pharmaceutical Benefits Scheme (PBS) website.
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient metformin
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: TIH-METFORMIN metformin hydrochloride 1000 mg tablet blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Metformin is indicated in the treatment of type 2 diabetes mellitus not satisfactorily controlled by diet, where the risk of lactic acidosis is minimised by excluding predisposing factors, especially impaired renal, hepatic or cardiovascular function. Metformin may be used as initial therapy or in sulfonylurea failure, either alone or in combination with a sulfonylurea or as adjuvant therapy in insulin-dependent diabetes.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, film coated
- Oral
- Oval, white tablet, embossed with M/O on one side and ">/>" on the other.
Images


Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 30 degrees Celsius
- Shelf lifetime is 3 Years.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 10 tablets pack
- 100 tablets pack
- 15 tablets pack
- 60 tablets pack
- 90 tablets pack
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient metformin
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: TIH-METFORMIN metformin hydrochloride 1000 mg tablet bottleConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Metformin is indicated in the treatment of type 2 diabetes mellitus not satisfactorily controlled by diet, where the risk of lactic acidosis is minimised by excluding predisposing factors, especially impaired renal, hepatic or cardiovascular function. Metformin may be used as initial therapy or in sulfonylurea failure, either alone or in combination with a sulfonylurea or as adjuvant therapy in insulin-dependent diabetes.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, film coated
- Oral
- Oval, white tablet, embossed with M/O on one side and ">/>" on the other.
Storage conditions
(ARTG)
- Store below 30 degrees Celsius
- Shelf lifetime is 3 Years.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 10 tablets pack
- 100 tablets pack
- 60 tablets pack
- 90 tablets pack
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient metformin
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: TIH-METFORMIN metformin hydrochloride 1000 mg tablet bottleConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Metformin is indicated in the treatment of type 2 diabetes mellitus not satisfactorily controlled by diet, where the risk of lactic acidosis is minimised by excluding predisposing factors, especially impaired renal, hepatic or cardiovascular function. Metformin may be used as initial therapy or in sulfonylurea failure, either alone or in combination with a sulfonylurea or as adjuvant therapy in insulin-dependent diabetes.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, film coated
- Oral
- Oval, white tablet, embossed with M/O on one side and ">/>" on the other.
Storage conditions
(ARTG)
- Store below 30 degrees Celsius
- Shelf lifetime is 3 Years.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 10 tablets pack
- 100 tablets pack
- 60 tablets pack
- 90 tablets pack
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient metformin
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: TIH-METFORMIN metformin hydrochloride 1000 mg tablet bottleConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Metformin is indicated in the treatment of type 2 diabetes mellitus not satisfactorily controlled by diet, where the risk of lactic acidosis is minimised by excluding predisposing factors, especially impaired renal, hepatic or cardiovascular function. Metformin may be used as initial therapy or in sulfonylurea failure, either alone or in combination with a sulfonylurea or as adjuvant therapy in insulin-dependent diabetes.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, film coated
- Oral
- Oval, white tablet, embossed with M/O on one side and ">/>" on the other.
Storage conditions
(ARTG)
- Store below 30 degrees Celsius
- Shelf lifetime is 3 Years.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 10 tablets pack
- 100 tablets pack
- 60 tablets pack
- 90 tablets pack
Is this medicine subsidised ?
(PBS)
This medicine was verified as being available on the PBS (Pharmaceutical Benefits Scheme) on January, 6 2026. To learn more about this subsidy, visit the Pharmaceutical Benefits Scheme (PBS) website.
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient metformin
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: TIH-METFORMIN metformin hydrochloride 1000 mg tablet bottleConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Metformin is indicated in the treatment of type 2 diabetes mellitus not satisfactorily controlled by diet, where the risk of lactic acidosis is minimised by excluding predisposing factors, especially impaired renal, hepatic or cardiovascular function. Metformin may be used as initial therapy or in sulfonylurea failure, either alone or in combination with a sulfonylurea or as adjuvant therapy in insulin-dependent diabetes.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, film coated
- Oral
- Oval, white tablet, embossed with M/O on one side and ">/>" on the other.
Storage conditions
(ARTG)
- Store below 30 degrees Celsius
- Shelf lifetime is 3 Years.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 10 tablets pack
- 100 tablets pack
- 60 tablets pack
- 90 tablets pack
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient metformin
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: TIH-METFORMIN metformin hydrochloride 500 mg tablet bottleConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Metformin is indicated in the treatment of type 2 diabetes mellitus not satisfactorily controlled by diet, where the risk of lactic acidosis is minimised by excluding predisposing factors, especially impaired renal, hepatic or cardiovascular function. Metformin may be used as initial therapy or in sulfonylurea failure, either alone or in combination with a sulfonylurea or as adjuvant therapy in insulin-dependent diabetes.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, film coated
- Oral
- Oblong, white tablet, embossed with M/O on one side and the Arrow symbol ">" on the other.
Storage conditions
(ARTG)
- Store below 30 degrees Celsius
- Shelf lifetime is 3 Years.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 10 tablets pack
- 100 tablets pack
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient metformin
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: TIH-METFORMIN metformin hydrochloride 500 mg tablet bottleConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Metformin is indicated in the treatment of type 2 diabetes mellitus not satisfactorily controlled by diet, where the risk of lactic acidosis is minimised by excluding predisposing factors, especially impaired renal, hepatic or cardiovascular function. Metformin may be used as initial therapy or in sulfonylurea failure, either alone or in combination with a sulfonylurea or as adjuvant therapy in insulin-dependent diabetes.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, film coated
- Oral
- Oblong, white tablet, embossed with M/O on one side and the Arrow symbol ">" on the other.
Storage conditions
(ARTG)
- Store below 30 degrees Celsius
- Shelf lifetime is 3 Years.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 10 tablets pack
- 100 tablets pack
Is this medicine subsidised ?
(PBS)
This medicine was verified as being available on the PBS (Pharmaceutical Benefits Scheme) on January, 6 2026. To learn more about this subsidy, visit the Pharmaceutical Benefits Scheme (PBS) website.
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient metformin
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: TIH-METFORMIN metformin hydrochloride 850 mg tablet bottleWhat this medicine is used for
(ARTG)
Metformin is indicated in the treatment of type 2 diabetes mellitus not satisfactorily controlled by diet, where the risk of lactic acidosis is minimised by excluding predisposing factors, especially impaired renal, hepatic or cardiovascular function. Metformin may be used as initial therapy or in sulfonylurea failure, either alone or in combination with a sulfonylurea or as adjuvant therapy in insulin-dependent diabetes.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, film coated
- Oral
- Round, white tablet (approx. 13mm), emobossed with MO on one side and the Arrow symbol ">" on the other.
Storage conditions
(ARTG)
- Store below 30 degrees Celsius
- Shelf lifetime is 3 Years.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 10 tablets - for dispensing only, not for individual patient supply pack
- 60 tablets - for dispensing only, not for individual patient supply pack
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient metformin
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: TIH-METFORMIN metformin hydrochloride 850 mg tablet bottleWhat this medicine is used for
(ARTG)
Metformin is indicated in the treatment of type 2 diabetes mellitus not satisfactorily controlled by diet, where the risk of lactic acidosis is minimised by excluding predisposing factors, especially impaired renal, hepatic or cardiovascular function. Metformin may be used as initial therapy or in sulfonylurea failure, either alone or in combination with a sulfonylurea or as adjuvant therapy in insulin-dependent diabetes.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, film coated
- Oral
- Round, white tablet (approx. 13mm), emobossed with MO on one side and the Arrow symbol ">" on the other.
Storage conditions
(ARTG)
- Store below 30 degrees Celsius
- Shelf lifetime is 3 Years.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 10 tablets - for dispensing only, not for individual patient supply pack
- 60 tablets - for dispensing only, not for individual patient supply pack
Is this medicine subsidised ?
(PBS)
This medicine was verified as being available on the PBS (Pharmaceutical Benefits Scheme) on January, 6 2026. To learn more about this subsidy, visit the Pharmaceutical Benefits Scheme (PBS) website.
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient metformin
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: APX-METFORMIN metformin hydrochloride 1000 mg tablet blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Metformin is indicated in the treatment of type 2 diabetes mellitus in adults, children from 10 years of age and adolescents, particularly in overweight patients, when dietary management and exercise alone does not result in adequate glycaemic control. For adult patients, metformin may be used as initial treatment or in sulfonylurea failures either alone or in combination with a sulfonylurea and other oral agents or as adjuvant therapy in insulin requiring type 2 diabetes.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, film coated
- Oral
- White to off white, oval shaped, biconvex tablets debossed with '10' and '00' on either side of deep notch on one side and breakline on other side
Images
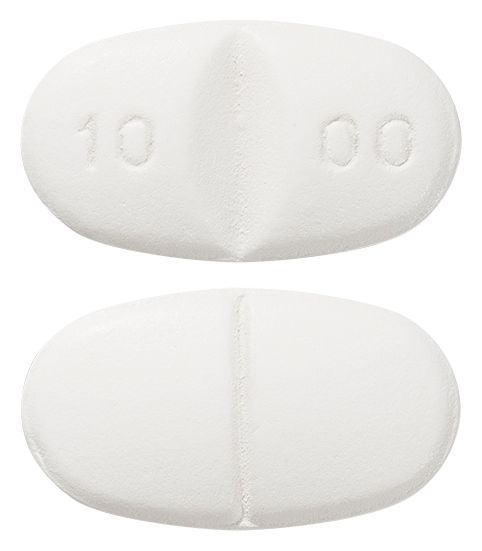

Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 25 degrees Celsius
- Shelf lifetime is 36 Months.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 10 pack
- 100 pack
- 14 pack
- 20 pack
- 28 pack
- 30 pack
- 56 pack
- 60 pack
- 90 pack
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient metformin
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: APX-METFORMIN metformin hydrochloride 1000 mg tablet blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Metformin is indicated in the treatment of type 2 diabetes mellitus in adults, children from 10 years of age and adolescents, particularly in overweight patients, when dietary management and exercise alone does not result in adequate glycaemic control. For adult patients, metformin may be used as initial treatment or in sulfonylurea failures either alone or in combination with a sulfonylurea and other oral agents or as adjuvant therapy in insulin requiring type 2 diabetes.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, film coated
- Oral
- White to off white, oval shaped, biconvex tablets debossed with '10' and '00' on either side of deep notch on one side and breakline on other side
Images


Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 25 degrees Celsius
- Shelf lifetime is 36 Months.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 10 pack
- 100 pack
- 14 pack
- 20 pack
- 28 pack
- 30 pack
- 56 pack
- 60 pack
- 90 pack
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient metformin
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: APX-METFORMIN metformin hydrochloride 500 mg tablet blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Metformin is indicated in the treatment of type 2 diabetes mellitus in adults, children from 10 years of age and adolescents, particularly in overweight patients, when dietary management and exercise alone does not result in adequate glycaemic control. For adult patients, metformin may be used as initial treatment or in sulfonylurea failures either alone or in combination with a sulfonylurea and other oral agents or as adjuvant therapy in insulin requiring type 2 diabetes.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, film coated
- Oral
- White coloured, film coated, round, biconvex tablets with 500 debossing
Images


Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 25 degrees Celsius
- Shelf lifetime is 36 Months.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 10 pack
- 100 pack
- 14 pack
- 20 pack
- 28 pack
- 30 pack
- 56 pack
- 60 pack
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient metformin
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: APX-METFORMIN metformin hydrochloride 500 mg tablet blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Metformin is indicated in the treatment of type 2 diabetes mellitus in adults, children from 10 years of age and adolescents, particularly in overweight patients, when dietary management and exercise alone does not result in adequate glycaemic control. For adult patients, metformin may be used as initial treatment or in sulfonylurea failures either alone or in combination with a sulfonylurea and other oral agents or as adjuvant therapy in insulin requiring type 2 diabetes.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, film coated
- Oral
- White coloured, film coated, round, biconvex tablets with 500 debossing
Images


Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 25 degrees Celsius
- Shelf lifetime is 36 Months.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 10 pack
- 100 pack
- 14 pack
- 20 pack
- 28 pack
- 30 pack
- 56 pack
- 60 pack
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient metformin
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: APX-METFORMIN metformin hydrochloride 500 mg tablet blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Metformin is indicated in the treatment of type 2 diabetes mellitus in adults, children from 10 years of age and adolescents, particularly in overweight patients, when dietary management and exercise alone does not result in adequate glycaemic control. For adult patients, metformin may be used as initial treatment or in sulfonylurea failures either alone or in combination with a sulfonylurea and other oral agents or as adjuvant therapy in insulin requiring type 2 diabetes.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, film coated
- Oral
- White coloured, film coated, round, biconvex tablets with 500 debossing
Images


Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 25 degrees Celsius
- Shelf lifetime is 36 Months.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 10 pack
- 100 pack
- 14 pack
- 20 pack
- 28 pack
- 30 pack
- 56 pack
- 60 pack
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient metformin
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: APX-METFORMIN metformin hydrochloride 850 mg tablet blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Metformin is indicated in the treatment of type 2 diabetes mellitus in adults, children from 10 years of age and adolescents, particularly in overweight patients, when dietary management and exercise alone does not result in adequate glycaemic control. For adult patients, metformin may be used as initial treatment or in sulfonylurea failures either alone or in combination with a sulfonylurea and other oral agents or as adjuvant therapy in insulin requiring type 2 diabetes.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, film coated
- Oral
- White coloured, film coated, round, biconvex tablets with 850 debossing
Images



Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 25 degrees Celsius
- Shelf lifetime is 36 Months.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 10 pack
- 100 pack
- 14 pack
- 20 pack
- 28 pack
- 30 pack
- 56 pack
- 60 pack
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient metformin
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: APX-METFORMIN metformin hydrochloride 850 mg tablet blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Metformin is indicated in the treatment of type 2 diabetes mellitus in adults, children from 10 years of age and adolescents, particularly in overweight patients, when dietary management and exercise alone does not result in adequate glycaemic control. For adult patients, metformin may be used as initial treatment or in sulfonylurea failures either alone or in combination with a sulfonylurea and other oral agents or as adjuvant therapy in insulin requiring type 2 diabetes.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, film coated
- Oral
- White coloured, film coated, round, biconvex tablets with 850 debossing
Images



Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 25 degrees Celsius
- Shelf lifetime is 36 Months.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 10 pack
- 100 pack
- 14 pack
- 20 pack
- 28 pack
- 30 pack
- 56 pack
- 60 pack
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient metformin
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: APX-METFORMIN metformin hydrochloride 850 mg tablet blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Metformin is indicated in the treatment of type 2 diabetes mellitus in adults, children from 10 years of age and adolescents, particularly in overweight patients, when dietary management and exercise alone does not result in adequate glycaemic control. For adult patients, metformin may be used as initial treatment or in sulfonylurea failures either alone or in combination with a sulfonylurea and other oral agents or as adjuvant therapy in insulin requiring type 2 diabetes.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, film coated
- Oral
- White coloured, film coated, round, biconvex tablets with 850 debossing
Images



Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 25 degrees Celsius
- Shelf lifetime is 36 Months.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 10 pack
- 100 pack
- 14 pack
- 20 pack
- 28 pack
- 30 pack
- 56 pack
- 60 pack
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient metformin
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: APX-METFORMIN metformin hydrochloride 1000 mg tablet blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Metformin is indicated in the treatment of type 2 diabetes mellitus in adults, children from 10 years of age and adolescents, particularly in overweight patients, when dietary management and exercise alone does not result in adequate glycaemic control. For adult patients, metformin may be used as initial treatment or in sulfonylurea failures either alone or in combination with a sulfonylurea and other oral agents or as adjuvant therapy in insulin requiring type 2 diabetes.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, film coated
- Oral
- White to off white, oval shaped, biconvex tablets debossed with '10' and '00' on either side of deep notch on one side and breakline on other side
Images


Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 25 degrees Celsius
- Shelf lifetime is 36 Months.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 10 pack
- 100 pack
- 14 pack
- 20 pack
- 28 pack
- 30 pack
- 56 pack
- 60 pack
- 90 pack
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient metformin
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: APX-METFORMIN metformin hydrochloride 1000 mg tablet blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Metformin is indicated in the treatment of type 2 diabetes mellitus in adults, children from 10 years of age and adolescents, particularly in overweight patients, when dietary management and exercise alone does not result in adequate glycaemic control. For adult patients, metformin may be used as initial treatment or in sulfonylurea failures either alone or in combination with a sulfonylurea and other oral agents or as adjuvant therapy in insulin requiring type 2 diabetes.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, film coated
- Oral
- White to off white, oval shaped, biconvex tablets debossed with '10' and '00' on either side of deep notch on one side and breakline on other side
Images


Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 25 degrees Celsius
- Shelf lifetime is 36 Months.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 10 pack
- 100 pack
- 14 pack
- 20 pack
- 28 pack
- 30 pack
- 56 pack
- 60 pack
- 90 pack
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient metformin
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: APX-METFORMIN metformin hydrochloride 500 mg tablet blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Metformin is indicated in the treatment of type 2 diabetes mellitus in adults, children from 10 years of age and adolescents, particularly in overweight patients, when dietary management and exercise alone does not result in adequate glycaemic control. For adult patients, metformin may be used as initial treatment or in sulfonylurea failures either alone or in combination with a sulfonylurea and other oral agents or as adjuvant therapy in insulin requiring type 2 diabetes.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, film coated
- Oral
- White coloured, film coated, round, biconvex tablets with 500 debossing
Images


Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 25 degrees Celsius
- Shelf lifetime is 36 Months.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 10 pack
- 100 pack
- 14 pack
- 20 pack
- 28 pack
- 30 pack
- 56 pack
- 60 pack
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient metformin
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: APX-METFORMIN metformin hydrochloride 500 mg tablet blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Metformin is indicated in the treatment of type 2 diabetes mellitus in adults, children from 10 years of age and adolescents, particularly in overweight patients, when dietary management and exercise alone does not result in adequate glycaemic control. For adult patients, metformin may be used as initial treatment or in sulfonylurea failures either alone or in combination with a sulfonylurea and other oral agents or as adjuvant therapy in insulin requiring type 2 diabetes.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, film coated
- Oral
- White coloured, film coated, round, biconvex tablets with 500 debossing
Images


Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 25 degrees Celsius
- Shelf lifetime is 36 Months.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 10 pack
- 100 pack
- 14 pack
- 20 pack
- 28 pack
- 30 pack
- 56 pack
- 60 pack
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient metformin
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: APX-METFORMIN metformin hydrochloride 850 mg tablet blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Metformin is indicated in the treatment of type 2 diabetes mellitus in adults, children from 10 years of age and adolescents, particularly in overweight patients, when dietary management and exercise alone does not result in adequate glycaemic control. For adult patients, metformin may be used as initial treatment or in sulfonylurea failures either alone or in combination with a sulfonylurea and other oral agents or as adjuvant therapy in insulin requiring type 2 diabetes.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, film coated
- Oral
- White coloured, film coated, round, biconvex tablets with 850 debossing
Images



Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 25 degrees Celsius
- Shelf lifetime is 36 Months.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 10 pack
- 100 pack
- 14 pack
- 20 pack
- 28 pack
- 30 pack
- 56 pack
- 60 pack
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient metformin
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: APX-METFORMIN metformin hydrochloride 1000 mg tablet blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Metformin is indicated in the treatment of type 2 diabetes mellitus in adults, children from 10 years of age and adolescents, particularly in overweight patients, when dietary management and exercise alone does not result in adequate glycaemic control. For adult patients, metformin may be used as initial treatment or in sulfonylurea failures either alone or in combination with a sulfonylurea and other oral agents or as adjuvant therapy in insulin requiring type 2 diabetes.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, film coated
- Oral
- White to off white, oval shaped, biconvex tablets debossed with '10' and '00' on either side of deep notch on one side and breakline on other side
Images


Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 25 degrees Celsius
- Shelf lifetime is 36 Months.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 10 pack
- 100 pack
- 14 pack
- 20 pack
- 28 pack
- 30 pack
- 56 pack
- 60 pack
- 90 pack
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient metformin
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: APX-METFORMIN metformin hydrochloride 850 mg tablet blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Metformin is indicated in the treatment of type 2 diabetes mellitus in adults, children from 10 years of age and adolescents, particularly in overweight patients, when dietary management and exercise alone does not result in adequate glycaemic control. For adult patients, metformin may be used as initial treatment or in sulfonylurea failures either alone or in combination with a sulfonylurea and other oral agents or as adjuvant therapy in insulin requiring type 2 diabetes.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, film coated
- Oral
- White coloured, film coated, round, biconvex tablets with 850 debossing
Images



Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 25 degrees Celsius
- Shelf lifetime is 36 Months.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 10 pack
- 100 pack
- 14 pack
- 20 pack
- 28 pack
- 30 pack
- 56 pack
- 60 pack
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient metformin
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: APX-METFORMIN metformin hydrochloride 850 mg tablet blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Metformin is indicated in the treatment of type 2 diabetes mellitus in adults, children from 10 years of age and adolescents, particularly in overweight patients, when dietary management and exercise alone does not result in adequate glycaemic control. For adult patients, metformin may be used as initial treatment or in sulfonylurea failures either alone or in combination with a sulfonylurea and other oral agents or as adjuvant therapy in insulin requiring type 2 diabetes.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, film coated
- Oral
- White coloured, film coated, round, biconvex tablets with 850 debossing
Images



Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 25 degrees Celsius
- Shelf lifetime is 36 Months.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 10 pack
- 100 pack
- 14 pack
- 20 pack
- 28 pack
- 30 pack
- 56 pack
- 60 pack
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient metformin
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems









