Ciclosporin (Apo) TM
You should seek medical advice in relation to medicines and use only as directed by a healthcare professional. Always read the label. If symptoms persist see your healthcare professional.
Active ingredients: ciclosporin
Brand name
(ARTG)
: APO-CICLOSPORIN ciclosporin 25 mg soft capsule blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Ciclosporin is indicated: As an immunosuppressive agent for the prevention of graft rejection following kidney, liver and heart allogeneic transplantation. For induction and/or maintenance of remission in the nephrotic syndrome. Ciclosporin is not a first-line agent. Its use should be restricted to occasions when steroids and cytostatic drugs have failed, or are not tolerated, or are considered inappropriate, and when renal function is unimpaired (see section 4.4 Special Warnings and Precautions for Use). For the treatment of severe, active rheumatoid arthritis in patients for whom classical slow-acting antirheumatic agents (including methotrexate) are inappropriate or ineffective. In patients with severe psoriasis in whom conventional therapy is ineffective or inappropriate and the disease has caused a significant interference with quality of life. For the treatment of severe atopic dermatitis when other treatment is ineffective or inappropriate.,Careful monitoring of all ciclosporin-treated patients is mandatory. Ciclosporin should only be used by medical practitioners who are experienced in the use of immunosuppressive therapy (see SECTION 4.4 SPECIAL WARNINGS AND PRECAUTIONS FOR USE).
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Capsule, soft
- Oral
- Grey oval soft gelatin capsules
Images


Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 25 degrees Celsius
- Protect from Light
- Store in Original Container
- Shelf lifetime is 24 Months.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 30 pack
- 50 pack
- 60 pack
Is this medicine subsidised ?
(PBS)
This medicine was verified as being available on the PBS (Pharmaceutical Benefits Scheme) on November, 1 2025. To learn more about this subsidy, visit the Pharmaceutical Benefits Scheme (PBS) website.
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient ciclosporin
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: APO-CICLOSPORIN ciclosporin 25 mg soft capsule blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Ciclosporin is indicated: As an immunosuppressive agent for the prevention of graft rejection following kidney, liver and heart allogeneic transplantation. For induction and/or maintenance of remission in the nephrotic syndrome. Ciclosporin is not a first-line agent. Its use should be restricted to occasions when steroids and cytostatic drugs have failed, or are not tolerated, or are considered inappropriate, and when renal function is unimpaired (see section 4.4 Special Warnings and Precautions for Use). For the treatment of severe, active rheumatoid arthritis in patients for whom classical slow-acting antirheumatic agents (including methotrexate) are inappropriate or ineffective. In patients with severe psoriasis in whom conventional therapy is ineffective or inappropriate and the disease has caused a significant interference with quality of life. For the treatment of severe atopic dermatitis when other treatment is ineffective or inappropriate.,Careful monitoring of all ciclosporin-treated patients is mandatory. Ciclosporin should only be used by medical practitioners who are experienced in the use of immunosuppressive therapy (see SECTION 4.4 SPECIAL WARNINGS AND PRECAUTIONS FOR USE).
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Capsule, soft
- Oral
- Grey oval soft gelatin capsules
Images


Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 25 degrees Celsius
- Protect from Light
- Store in Original Container
- Shelf lifetime is 24 Months.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 30 pack
- 50 pack
- 60 pack
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient ciclosporin
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: APO-CICLOSPORIN ciclosporin 25 mg soft capsule blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Ciclosporin is indicated: As an immunosuppressive agent for the prevention of graft rejection following kidney, liver and heart allogeneic transplantation. For induction and/or maintenance of remission in the nephrotic syndrome. Ciclosporin is not a first-line agent. Its use should be restricted to occasions when steroids and cytostatic drugs have failed, or are not tolerated, or are considered inappropriate, and when renal function is unimpaired (see section 4.4 Special Warnings and Precautions for Use). For the treatment of severe, active rheumatoid arthritis in patients for whom classical slow-acting antirheumatic agents (including methotrexate) are inappropriate or ineffective. In patients with severe psoriasis in whom conventional therapy is ineffective or inappropriate and the disease has caused a significant interference with quality of life. For the treatment of severe atopic dermatitis when other treatment is ineffective or inappropriate.,Careful monitoring of all ciclosporin-treated patients is mandatory. Ciclosporin should only be used by medical practitioners who are experienced in the use of immunosuppressive therapy (see SECTION 4.4 SPECIAL WARNINGS AND PRECAUTIONS FOR USE).
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Capsule, soft
- Oral
- Grey oval soft gelatin capsules
Images


Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 25 degrees Celsius
- Protect from Light
- Store in Original Container
- Shelf lifetime is 24 Months.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 30 pack
- 50 pack
- 60 pack
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient ciclosporin
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: APO-CICLOSPORIN ciclosporin 100 mg soft capsule blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Ciclosporin is indicated: As an immunosuppressive agent for the prevention of graft rejection following kidney, liver and heart allogeneic transplantation. For induction and/or maintenance of remission in the nephrotic syndrome. Ciclosporin is not a first-line agent. Its use should be restricted to occasions when steroids and cytostatic drugs have failed, or are not tolerated, or are considered inappropriate, and when renal function is unimpaired (see section 4.4 Special Warnings and Precautions for Use). For the treatment of severe, active rheumatoid arthritis in patients for whom classical slow-acting antirheumatic agents (including methotrexate) are inappropriate or ineffective. In patients with severe psoriasis in whom conventional therapy is ineffective or inappropriate and the disease has caused a significant interference with quality of life. For the treatment of severe atopic dermatitis when other treatment is ineffective or inappropriate.,Careful monitoring of all ciclosporin-treated patients is mandatory. Ciclosporin should only be used by medical practitioners who are experienced in the use of immunosuppressive therapy (see SECTION 4.4 SPECIAL WARNINGS AND PRECAUTIONS FOR USE).
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Capsule, soft
- Oral
- Grey oblong soft gelatin capsules
Images
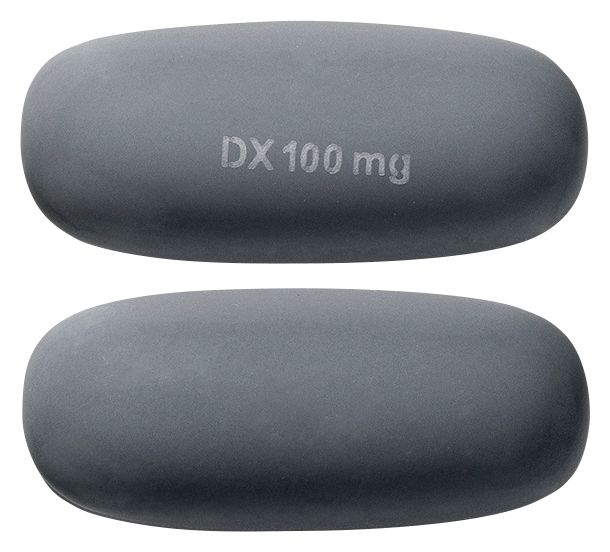

Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 25 degrees Celsius
- Protect from Light
- Store in Original Container
- Shelf lifetime is 21 Months.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 30 pack
- 50 pack
- 60 pack
Is this medicine subsidised ?
(PBS)
This medicine was verified as being available on the PBS (Pharmaceutical Benefits Scheme) on November, 1 2025. To learn more about this subsidy, visit the Pharmaceutical Benefits Scheme (PBS) website.
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient ciclosporin
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: APO-CICLOSPORIN ciclosporin 100 mg soft capsule blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Ciclosporin is indicated: As an immunosuppressive agent for the prevention of graft rejection following kidney, liver and heart allogeneic transplantation. For induction and/or maintenance of remission in the nephrotic syndrome. Ciclosporin is not a first-line agent. Its use should be restricted to occasions when steroids and cytostatic drugs have failed, or are not tolerated, or are considered inappropriate, and when renal function is unimpaired (see section 4.4 Special Warnings and Precautions for Use). For the treatment of severe, active rheumatoid arthritis in patients for whom classical slow-acting antirheumatic agents (including methotrexate) are inappropriate or ineffective. In patients with severe psoriasis in whom conventional therapy is ineffective or inappropriate and the disease has caused a significant interference with quality of life. For the treatment of severe atopic dermatitis when other treatment is ineffective or inappropriate.,Careful monitoring of all ciclosporin-treated patients is mandatory. Ciclosporin should only be used by medical practitioners who are experienced in the use of immunosuppressive therapy (see SECTION 4.4 SPECIAL WARNINGS AND PRECAUTIONS FOR USE).
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Capsule, soft
- Oral
- Grey oblong soft gelatin capsules
Images


Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 25 degrees Celsius
- Protect from Light
- Store in Original Container
- Shelf lifetime is 21 Months.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 30 pack
- 50 pack
- 60 pack
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient ciclosporin
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: APO-CICLOSPORIN ciclosporin 100 mg soft capsule blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Ciclosporin is indicated: As an immunosuppressive agent for the prevention of graft rejection following kidney, liver and heart allogeneic transplantation. For induction and/or maintenance of remission in the nephrotic syndrome. Ciclosporin is not a first-line agent. Its use should be restricted to occasions when steroids and cytostatic drugs have failed, or are not tolerated, or are considered inappropriate, and when renal function is unimpaired (see section 4.4 Special Warnings and Precautions for Use). For the treatment of severe, active rheumatoid arthritis in patients for whom classical slow-acting antirheumatic agents (including methotrexate) are inappropriate or ineffective. In patients with severe psoriasis in whom conventional therapy is ineffective or inappropriate and the disease has caused a significant interference with quality of life. For the treatment of severe atopic dermatitis when other treatment is ineffective or inappropriate.,Careful monitoring of all ciclosporin-treated patients is mandatory. Ciclosporin should only be used by medical practitioners who are experienced in the use of immunosuppressive therapy (see SECTION 4.4 SPECIAL WARNINGS AND PRECAUTIONS FOR USE).
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Capsule, soft
- Oral
- Grey oblong soft gelatin capsules
Images


Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 25 degrees Celsius
- Protect from Light
- Store in Original Container
- Shelf lifetime is 21 Months.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 30 pack
- 50 pack
- 60 pack
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient ciclosporin
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: APO-CICLOSPORIN ciclosporin 50 mg soft capsule blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Ciclosporin is indicated: As an immunosuppressive agent for the prevention of graft rejection following kidney, liver and heart allogeneic transplantation. For induction and/or maintenance of remission in the nephrotic syndrome. Ciclosporin is not a first-line agent. Its use should be restricted to occasions when steroids and cytostatic drugs have failed, or are not tolerated, or are considered inappropriate, and when renal function is unimpaired (see section 4.4 Special Warnings and Precautions for Use). For the treatment of severe, active rheumatoid arthritis in patients for whom classical slow-acting antirheumatic agents (including methotrexate) are inappropriate or ineffective. In patients with severe psoriasis in whom conventional therapy is ineffective or inappropriate and the disease has caused a significant interference with quality of life. For the treatment of severe atopic dermatitis when other treatment is ineffective or inappropriate.,Careful monitoring of all ciclosporin-treated patients is mandatory. Ciclosporin should only be used by medical practitioners who are experienced in the use of immunosuppressive therapy (see SECTION 4.4 SPECIAL WARNINGS AND PRECAUTIONS FOR USE).
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Capsule, soft
- Oral
- Grey oblong soft gelatin capsules
Images
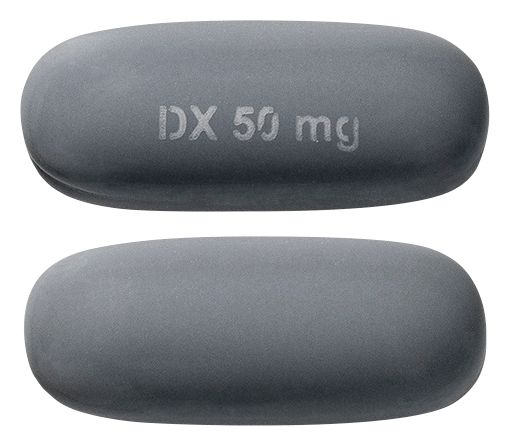

Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 25 degrees Celsius
- Protect from Light
- Store in Original Container
- Shelf lifetime is 24 Months.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 30 pack
- 50 pack
- 60 pack
Is this medicine subsidised ?
(PBS)
This medicine was verified as being available on the PBS (Pharmaceutical Benefits Scheme) on November, 1 2025. To learn more about this subsidy, visit the Pharmaceutical Benefits Scheme (PBS) website.
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient ciclosporin
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: APO-CICLOSPORIN ciclosporin 50 mg soft capsule blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Ciclosporin is indicated: As an immunosuppressive agent for the prevention of graft rejection following kidney, liver and heart allogeneic transplantation. For induction and/or maintenance of remission in the nephrotic syndrome. Ciclosporin is not a first-line agent. Its use should be restricted to occasions when steroids and cytostatic drugs have failed, or are not tolerated, or are considered inappropriate, and when renal function is unimpaired (see section 4.4 Special Warnings and Precautions for Use). For the treatment of severe, active rheumatoid arthritis in patients for whom classical slow-acting antirheumatic agents (including methotrexate) are inappropriate or ineffective. In patients with severe psoriasis in whom conventional therapy is ineffective or inappropriate and the disease has caused a significant interference with quality of life. For the treatment of severe atopic dermatitis when other treatment is ineffective or inappropriate.,Careful monitoring of all ciclosporin-treated patients is mandatory. Ciclosporin should only be used by medical practitioners who are experienced in the use of immunosuppressive therapy (see SECTION 4.4 SPECIAL WARNINGS AND PRECAUTIONS FOR USE).
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Capsule, soft
- Oral
- Grey oblong soft gelatin capsules
Images


Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 25 degrees Celsius
- Protect from Light
- Store in Original Container
- Shelf lifetime is 24 Months.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 30 pack
- 50 pack
- 60 pack
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient ciclosporin
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: APO-CICLOSPORIN ciclosporin 50 mg soft capsule blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Ciclosporin is indicated: As an immunosuppressive agent for the prevention of graft rejection following kidney, liver and heart allogeneic transplantation. For induction and/or maintenance of remission in the nephrotic syndrome. Ciclosporin is not a first-line agent. Its use should be restricted to occasions when steroids and cytostatic drugs have failed, or are not tolerated, or are considered inappropriate, and when renal function is unimpaired (see section 4.4 Special Warnings and Precautions for Use). For the treatment of severe, active rheumatoid arthritis in patients for whom classical slow-acting antirheumatic agents (including methotrexate) are inappropriate or ineffective. In patients with severe psoriasis in whom conventional therapy is ineffective or inappropriate and the disease has caused a significant interference with quality of life. For the treatment of severe atopic dermatitis when other treatment is ineffective or inappropriate.,Careful monitoring of all ciclosporin-treated patients is mandatory. Ciclosporin should only be used by medical practitioners who are experienced in the use of immunosuppressive therapy (see SECTION 4.4 SPECIAL WARNINGS AND PRECAUTIONS FOR USE).
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Capsule, soft
- Oral
- Grey oblong soft gelatin capsules
Images


Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 25 degrees Celsius
- Protect from Light
- Store in Original Container
- Shelf lifetime is 24 Months.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 30 pack
- 50 pack
- 60 pack
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient ciclosporin
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems









