Provera TM
Listen to the Pronunciation:
You should seek medical advice in relation to medicines and use only as directed by a healthcare professional. Always read the label. If symptoms persist see your healthcare professional.
Active ingredients: medroxyprogesterone
Brand name
(ARTG)
: PROVERA medroxyprogesterone acetate 100mg tablet blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
Listen to the audio transcription of the CMI leaflet.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Carcinoma,Palliative treatment of recurrent and/or metastatic breast or renal cell cancer and of inoperable recurrent or metastatic endometrial carcinoma.,Endometriosis,For use in the treatment of visually proven (laparoscopy) endometriosis where the required end-point of treatment is pregnancy, or for the control of symptoms when surgery is contraindicated or has been unsuccessful.,Secondary amenorrhoea proven not due to pregnancy,In amenorrhoea associated with a poorly developed proliferative endometrium, conventional estrogen therapy may be employed in conjunction with MPA.,Abnormal uterine bleeding in the absence of organic pathology,Adjunct to estrogen therapy,Combination hormone replacement therapy, now referred to as menopausal hormone therapy (MHT), should only be used in non-hysterectomised women (see Section 4.4 Special warnings and precautions for use).
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, uncoated
- Oral
- white scored tablet marked "U467"
Images


Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 30 degrees Celsius
- Shelf lifetime is 3 Years.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 10 pack
- 100 pack
Is this medicine subsidised ?
(PBS)
This medicine was verified as being available on the PBS (Pharmaceutical Benefits Scheme) on January, 6 2026. To learn more about this subsidy, visit the Pharmaceutical Benefits Scheme (PBS) website.
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient medroxyprogesterone
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: PROVERA medroxyprogesterone acetate 250mg tablet blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
Listen to the audio transcription of the CMI leaflet.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Carcinoma,Palliative treatment of recurrent and/or metastatic breast or renal cell cancer and of inoperable recurrent or metastatic endometrial carcinoma.,Endometriosis,For use in the treatment of visually proven (laparoscopy) endometriosis where the required end-point of treatment is pregnancy, or for the control of symptoms when surgery is contraindicated or has been unsuccessful.,Secondary amenorrhoea proven not due to pregnancy,In amenorrhoea associated with a poorly developed proliferative endometrium, conventional estrogen therapy may be employed in conjunction with MPA.,Abnormal uterine bleeding in the absence of organic pathology,Adjunct to estrogen therapy,Combination hormone replacement therapy, now referred to as menopausal hormone therapy (MHT), should only be used in non-hysterectomised women (see Section 4.4 Special warnings and precautions for use).
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, uncoated
- Oral
- white scored tablet marked "U403"
Images
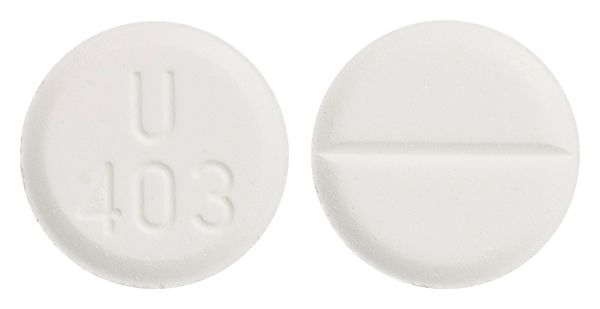

Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 30 degrees Celsius
- Shelf lifetime is 3 Years.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 10 pack
- 60 pack
Is this medicine subsidised ?
(PBS)
This medicine was verified as being available on the PBS (Pharmaceutical Benefits Scheme) on January, 6 2026. To learn more about this subsidy, visit the Pharmaceutical Benefits Scheme (PBS) website.
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient medroxyprogesterone
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: PROVERA medroxyprogesterone acetate 500mg tablet blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
Listen to the audio transcription of the CMI leaflet.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Carcinoma,Palliative treatment of recurrent and/or metastatic breast or renal cell cancer and of inoperable recurrent or metastatic endometrial carcinoma.,Endometriosis,For use in the treatment of visually proven (laparoscopy) endometriosis where the required end-point of treatment is pregnancy, or for the control of symptoms when surgery is contraindicated or has been unsuccessful.,Secondary amenorrhoea proven not due to pregnancy,In amenorrhoea associated with a poorly developed proliferative endometrium, conventional estrogen therapy may be employed in conjunction with MPA.,Abnormal uterine bleeding in the absence of organic pathology,Adjunct to estrogen therapy,Combination hormone replacement therapy, now referred to as menopausal hormone therapy (MHT), should only be used in non-hysterectomised women (see Section 4.4 Special warnings and precautions for use).
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, uncoated
- Oral
- white capsule shaped tablet marked "UPJOHN 717"
Images
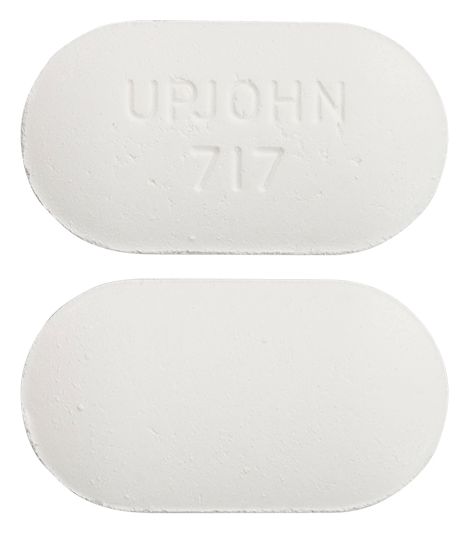

Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 30 degrees Celsius
- Shelf lifetime is 3 Years.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 10 pack
- 30 pack
- 60 pack
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient medroxyprogesterone
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: PROVERA medroxyprogesterone acetate 200mg tablet blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
Listen to the audio transcription of the CMI leaflet.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Carcinoma,Palliative treatment of recurrent and/or metastatic breast or renal cell cancer and of inoperable recurrent or metastatic endometrial carcinoma.,Endometriosis,For use in the treatment of visually proven (laparoscopy) endometriosis where the required end-point of treatment is pregnancy, or for the control of symptoms when surgery is contraindicated or has been unsuccessful.,Secondary amenorrhoea proven not due to pregnancy,In amenorrhoea associated with a poorly developed proliferative endometrium, conventional estrogen therapy may be employed in conjunction with MPA.,Abnormal uterine bleeding in the absence of organic pathology,Adjunct to estrogen therapy,Combination hormone replacement therapy, now referred to as menopausal hormone therapy (MHT), should only be used in non-hysterectomised women (see Section 4.4 Special warnings and precautions for use).
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, uncoated
- Oral
- white scored tablet marked "U320"
Images


Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 30 degrees Celsius
- Shelf lifetime is 3 Years.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 10 pack
- 60 pack
Is this medicine subsidised ?
(PBS)
This medicine was verified as being available on the PBS (Pharmaceutical Benefits Scheme) on January, 6 2026. To learn more about this subsidy, visit the Pharmaceutical Benefits Scheme (PBS) website.
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient medroxyprogesterone
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: PROVERA Medroxyprogesterone acetate 2.5mg tablet blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
Listen to the audio transcription of the CMI leaflet.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Carcinoma,Palliative treatment of recurrent and/or metastatic breast or renal cell cancer and of inoperable recurrent or metastatic endometrial carcinoma.,Endometriosis,For use in the treatment of visually proven (laparoscopy) endometriosis where the required end-point of treatment is pregnancy, or for the control of symptoms when surgery is contraindicated or has been unsuccessful.,Secondary amenorrhoea proven not due to pregnancy,In amenorrhoea associated with a poorly developed proliferative endometrium, conventional estrogen therapy may be employed in conjunction with MPA.,Abnormal uterine bleeding in the absence of organic pathology,Adjunct to estrogen therapy,Combination hormone replacement therapy, now referred to as menopausal hormone therapy (MHT), should only be used in non-hysterectomised women (see Section 4.4 Special warnings and precautions for use).
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, uncoated
- Oral
- Orange, circular (about 6.4mm diameter) tablets embossed "U64" on the opposite side to the score line..
Images


Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 30 degrees Celsius
- Shelf lifetime is 3 Years.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 28 tablets pack
- 56 tablets pack
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient medroxyprogesterone
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: PROVERA Medroxyprogesterone acetate 2.5mg tablet blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
Listen to the audio transcription of the CMI leaflet.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Carcinoma,Palliative treatment of recurrent and/or metastatic breast or renal cell cancer and of inoperable recurrent or metastatic endometrial carcinoma.,Endometriosis,For use in the treatment of visually proven (laparoscopy) endometriosis where the required end-point of treatment is pregnancy, or for the control of symptoms when surgery is contraindicated or has been unsuccessful.,Secondary amenorrhoea proven not due to pregnancy,In amenorrhoea associated with a poorly developed proliferative endometrium, conventional estrogen therapy may be employed in conjunction with MPA.,Abnormal uterine bleeding in the absence of organic pathology,Adjunct to estrogen therapy,Combination hormone replacement therapy, now referred to as menopausal hormone therapy (MHT), should only be used in non-hysterectomised women (see Section 4.4 Special warnings and precautions for use).
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, uncoated
- Oral
- Orange, circular (about 6.4mm diameter) tablets embossed "U64" on the opposite side to the score line..
Images


Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 30 degrees Celsius
- Shelf lifetime is 3 Years.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 28 tablets pack
- 56 tablets pack
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient medroxyprogesterone
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: PROVERA Medroxyprogesterone acetate 5mg tablet blister pack (New Formula)Consumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
Listen to the audio transcription of the CMI leaflet.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Carcinoma,Palliative treatment of recurrent and/or metastatic breast or renal cell cancer and of inoperable recurrent or metastatic endometrial carcinoma.,Endometriosis,For use in the treatment of visually proven (laparoscopy) endometriosis where the required end-point of treatment is pregnancy, or for the control of symptoms when surgery is contraindicated or has been unsuccessful.,Secondary amenorrhoea proven not due to pregnancy,In amenorrhoea associated with a poorly developed proliferative endometrium, conventional estrogen therapy may be employed in conjunction with MPA.,Abnormal uterine bleeding in the absence of organic pathology,Adjunct to estrogen therapy,Combination hormone replacement therapy, now referred to as menopausal hormone therapy (MHT), should only be used in non-hysterectomised women (see Section 4.4 Special warnings and precautions for use).
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, uncoated
- Oral
- Pale blue, round tablets. One surface is engraved with the logo "286" on both sides of a break-score. The other surface is engraved with the logo "U"
Images


Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 30 degrees Celsius
- Shelf lifetime is 3 Years.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 28 and 56 tablets pack
Is this medicine subsidised ?
(PBS)
This medicine was verified as being available on the PBS (Pharmaceutical Benefits Scheme) on January, 6 2026. To learn more about this subsidy, visit the Pharmaceutical Benefits Scheme (PBS) website.
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient medroxyprogesterone
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: PROVERA Medroxyprogesterone acetate 10mg tablet blister pack (new embossing)Consumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
Listen to the audio transcription of the CMI leaflet.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Carcinoma,Palliative treatment of recurrent and/or metastatic breast or renal cell cancer and of inoperable recurrent or metastatic endometrial carcinoma.,Endometriosis,For use in the treatment of visually proven (laparoscopy) endometriosis where the required end-point of treatment is pregnancy, or for the control of symptoms when surgery is contraindicated or has been unsuccessful.,Secondary amenorrhoea proven not due to pregnancy,In amenorrhoea associated with a poorly developed proliferative endometrium, conventional estrogen therapy may be employed in conjunction with MPA.,Abnormal uterine bleeding in the absence of organic pathology,Adjunct to estrogen therapy,Combination hormone replacement therapy, now referred to as menopausal hormone therapy (MHT), should only be used in non-hysterectomised women (see Section 4.4 Special warnings and precautions for use).
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, uncoated
- Oral
- White, circular (about 7.14mm diameter) tablets embossed "Upjohn 50" on one side and single scored on the other.
Images


Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 30 degrees Celsius
- Shelf lifetime is 3 Years.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 15 tablets pack
- 30 tablets pack
Is this medicine subsidised ?
(PBS)
This medicine was verified as being available on the PBS (Pharmaceutical Benefits Scheme) on January, 6 2026. To learn more about this subsidy, visit the Pharmaceutical Benefits Scheme (PBS) website.
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient medroxyprogesterone
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: PROVERA Medroxyprogesterone acetate 10mg tablet blister pack (new embossing)Consumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
Listen to the audio transcription of the CMI leaflet.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Carcinoma,Palliative treatment of recurrent and/or metastatic breast or renal cell cancer and of inoperable recurrent or metastatic endometrial carcinoma.,Endometriosis,For use in the treatment of visually proven (laparoscopy) endometriosis where the required end-point of treatment is pregnancy, or for the control of symptoms when surgery is contraindicated or has been unsuccessful.,Secondary amenorrhoea proven not due to pregnancy,In amenorrhoea associated with a poorly developed proliferative endometrium, conventional estrogen therapy may be employed in conjunction with MPA.,Abnormal uterine bleeding in the absence of organic pathology,Adjunct to estrogen therapy,Combination hormone replacement therapy, now referred to as menopausal hormone therapy (MHT), should only be used in non-hysterectomised women (see Section 4.4 Special warnings and precautions for use).
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, uncoated
- Oral
- White, circular (about 7.14mm diameter) tablets embossed "Upjohn 50" on one side and single scored on the other.
Images


Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 30 degrees Celsius
- Shelf lifetime is 3 Years.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 15 tablets pack
- 30 tablets pack
Is this medicine subsidised ?
(PBS)
This medicine was verified as being available on the PBS (Pharmaceutical Benefits Scheme) on January, 6 2026. To learn more about this subsidy, visit the Pharmaceutical Benefits Scheme (PBS) website.
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient medroxyprogesterone
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: PROVERA Medroxyprogesterone acetate 10mg tablet bottle (new embossing)Consumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
Listen to the audio transcription of the CMI leaflet.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Carcinoma,Palliative treatment of recurrent and/or metastatic breast or renal cell cancer and of inoperable recurrent or metastatic endometrial carcinoma.,Endometriosis,For use in the treatment of visually proven (laparoscopy) endometriosis where the required end-point of treatment is pregnancy, or for the control of symptoms when surgery is contraindicated or has been unsuccessful.,Secondary amenorrhoea proven not due to pregnancy,In amenorrhoea associated with a poorly developed proliferative endometrium, conventional estrogen therapy may be employed in conjunction with MPA.,Abnormal uterine bleeding in the absence of organic pathology,Adjunct to estrogen therapy,Combination hormone replacement therapy, now referred to as menopausal hormone therapy (MHT), should only be used in non-hysterectomised women (see Section 4.4 Special warnings and precautions for use).
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, uncoated
- Oral
- White, circular (about 7.14mm diameter) tablets embossed "Upjohn 50" on one side and single scored on the other.
Images


Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 30 degrees Celsius
- Shelf lifetime is 5 Years.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 100 tablets pack
Is this medicine subsidised ?
(PBS)
This medicine was verified as being available on the PBS (Pharmaceutical Benefits Scheme) on January, 6 2026. To learn more about this subsidy, visit the Pharmaceutical Benefits Scheme (PBS) website.
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient medroxyprogesterone
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: PROVERA medroxyprogesterone acetate 500mg tablet blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
Listen to the audio transcription of the CMI leaflet.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Carcinoma,Palliative treatment of recurrent and/or metastatic breast or renal cell cancer and of inoperable recurrent or metastatic endometrial carcinoma.,Endometriosis,For use in the treatment of visually proven (laparoscopy) endometriosis where the required end-point of treatment is pregnancy, or for the control of symptoms when surgery is contraindicated or has been unsuccessful.,Secondary amenorrhoea proven not due to pregnancy,In amenorrhoea associated with a poorly developed proliferative endometrium, conventional estrogen therapy may be employed in conjunction with MPA.,Abnormal uterine bleeding in the absence of organic pathology,Adjunct to estrogen therapy,Combination hormone replacement therapy, now referred to as menopausal hormone therapy (MHT), should only be used in non-hysterectomised women (see Section 4.4 Special warnings and precautions for use).
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, uncoated
- Oral
- white capsule shaped tablet marked "UPJOHN 717"
Images


Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 30 degrees Celsius
- Shelf lifetime is 3 Years.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 10 pack
- 30 pack
- 60 pack
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient medroxyprogesterone
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: PROVERA Medroxyprogesterone acetate 5mg tablet blister pack (New Formula)Consumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
Listen to the audio transcription of the CMI leaflet.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Carcinoma,Palliative treatment of recurrent and/or metastatic breast or renal cell cancer and of inoperable recurrent or metastatic endometrial carcinoma.,Endometriosis,For use in the treatment of visually proven (laparoscopy) endometriosis where the required end-point of treatment is pregnancy, or for the control of symptoms when surgery is contraindicated or has been unsuccessful.,Secondary amenorrhoea proven not due to pregnancy,In amenorrhoea associated with a poorly developed proliferative endometrium, conventional estrogen therapy may be employed in conjunction with MPA.,Abnormal uterine bleeding in the absence of organic pathology,Adjunct to estrogen therapy,Combination hormone replacement therapy, now referred to as menopausal hormone therapy (MHT), should only be used in non-hysterectomised women (see Section 4.4 Special warnings and precautions for use).
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, uncoated
- Oral
- Pale blue, round tablets. One surface is engraved with the logo "286" on both sides of a break-score. The other surface is engraved with the logo "U"
Images


Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 30 degrees Celsius
- Shelf lifetime is 3 Years.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 28 and 56 tablets pack
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient medroxyprogesterone
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: PROVERA Medroxyprogesterone acetate 10mg tablet blister pack (new embossing)Consumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
Listen to the audio transcription of the CMI leaflet.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Carcinoma,Palliative treatment of recurrent and/or metastatic breast or renal cell cancer and of inoperable recurrent or metastatic endometrial carcinoma.,Endometriosis,For use in the treatment of visually proven (laparoscopy) endometriosis where the required end-point of treatment is pregnancy, or for the control of symptoms when surgery is contraindicated or has been unsuccessful.,Secondary amenorrhoea proven not due to pregnancy,In amenorrhoea associated with a poorly developed proliferative endometrium, conventional estrogen therapy may be employed in conjunction with MPA.,Abnormal uterine bleeding in the absence of organic pathology,Adjunct to estrogen therapy,Combination hormone replacement therapy, now referred to as menopausal hormone therapy (MHT), should only be used in non-hysterectomised women (see Section 4.4 Special warnings and precautions for use).
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, uncoated
- Oral
- White, circular (about 7.14mm diameter) tablets embossed "Upjohn 50" on one side and single scored on the other.
Images


Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 30 degrees Celsius
- Shelf lifetime is 3 Years.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 15 tablets pack
- 30 tablets pack
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient medroxyprogesterone
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: PROVERA medroxyprogesterone acetate 250mg tablet blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
Listen to the audio transcription of the CMI leaflet.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Carcinoma,Palliative treatment of recurrent and/or metastatic breast or renal cell cancer and of inoperable recurrent or metastatic endometrial carcinoma.,Endometriosis,For use in the treatment of visually proven (laparoscopy) endometriosis where the required end-point of treatment is pregnancy, or for the control of symptoms when surgery is contraindicated or has been unsuccessful.,Secondary amenorrhoea proven not due to pregnancy,In amenorrhoea associated with a poorly developed proliferative endometrium, conventional estrogen therapy may be employed in conjunction with MPA.,Abnormal uterine bleeding in the absence of organic pathology,Adjunct to estrogen therapy,Combination hormone replacement therapy, now referred to as menopausal hormone therapy (MHT), should only be used in non-hysterectomised women (see Section 4.4 Special warnings and precautions for use).
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, uncoated
- Oral
- white scored tablet marked "U403"
Images


Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 30 degrees Celsius
- Shelf lifetime is 3 Years.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 10 pack
- 60 pack
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient medroxyprogesterone
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: PROVERA medroxyprogesterone acetate 500mg tablet blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
Listen to the audio transcription of the CMI leaflet.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Carcinoma,Palliative treatment of recurrent and/or metastatic breast or renal cell cancer and of inoperable recurrent or metastatic endometrial carcinoma.,Endometriosis,For use in the treatment of visually proven (laparoscopy) endometriosis where the required end-point of treatment is pregnancy, or for the control of symptoms when surgery is contraindicated or has been unsuccessful.,Secondary amenorrhoea proven not due to pregnancy,In amenorrhoea associated with a poorly developed proliferative endometrium, conventional estrogen therapy may be employed in conjunction with MPA.,Abnormal uterine bleeding in the absence of organic pathology,Adjunct to estrogen therapy,Combination hormone replacement therapy, now referred to as menopausal hormone therapy (MHT), should only be used in non-hysterectomised women (see Section 4.4 Special warnings and precautions for use).
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, uncoated
- Oral
- white capsule shaped tablet marked "UPJOHN 717"
Images


Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 30 degrees Celsius
- Shelf lifetime is 3 Years.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 10 pack
- 30 pack
- 60 pack
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient medroxyprogesterone
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: PROVERA medroxyprogesterone acetate 200mg tablet blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
Listen to the audio transcription of the CMI leaflet.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Carcinoma,Palliative treatment of recurrent and/or metastatic breast or renal cell cancer and of inoperable recurrent or metastatic endometrial carcinoma.,Endometriosis,For use in the treatment of visually proven (laparoscopy) endometriosis where the required end-point of treatment is pregnancy, or for the control of symptoms when surgery is contraindicated or has been unsuccessful.,Secondary amenorrhoea proven not due to pregnancy,In amenorrhoea associated with a poorly developed proliferative endometrium, conventional estrogen therapy may be employed in conjunction with MPA.,Abnormal uterine bleeding in the absence of organic pathology,Adjunct to estrogen therapy,Combination hormone replacement therapy, now referred to as menopausal hormone therapy (MHT), should only be used in non-hysterectomised women (see Section 4.4 Special warnings and precautions for use).
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, uncoated
- Oral
- white scored tablet marked "U320"
Images


Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 30 degrees Celsius
- Shelf lifetime is 3 Years.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 10 pack
- 60 pack
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient medroxyprogesterone
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems









