Kalma TM
Listen to the Pronunciation:
You should seek medical advice in relation to medicines and use only as directed by a healthcare professional. Always read the label. If symptoms persist see your healthcare professional.
Active ingredients: alprazolam
Brand name
(ARTG)
: KALMA 0.25 alprazolam 0.25mg tablet bottleWhat this medicine is used for
(ARTG)
For short-term symptomatic treatment of anxiety including treatment of anxious patients with some symptoms of depression. Alprazolam is not recommended as primary therapy in patients with depression. For the treatment of panic disorder with or without some phobic avoidance, and for blocking or attenuation of panic attacks and phobias in patients who have agoraphobia with panic attacks.INDICATIONS AS AT 17 DECEMBER 2001 : Anxiety. Short-term symptomatic treatment of anxiety including treatment of anxious patients with some symptoms of depression. Panic disorder. The treatment of panic disorder with or without some phobic avoidance, and for blocking or attenuation of panic attacks and phobias in patients who have agoraphobia with panic attacks. The diagnostic criteria for panic disorder in DSM-III-R are as follows: The panic attacks (discrete periods of intense fear or discomfort), at least initially, are unexpected. Later in the course of this disturbance, certain situations (e.g. driving a car or being in a crowded place) may become associated with having a panic attack. These panic attacks are not triggered by situations in which the person is the focus of others' attention (as in social phobia). The diagnosis requires four such attacks within a four week period, or one or more attacks followed by at least a month of persistent fear of having another attack. The panic attacks must be characterised by at least four of the following symptoms: dyspnoea or smothering sensations; dizziness, unsteady feelings or faintness; palpitations or tachycardia; trembling or shaking; sweating; choking; nausea or abdominal distress; depersonalisation or derealisation; paraesthesiae; flushes (hot flashes) or chills; chest pain or discomfort; fear of dying; fear of going crazy or of doing something uncontrolled. Note. Attacks involving four or more symptoms are panic attacks; attacks involving fewer than four are limited symptom attacks. At least some of the panic attack symptoms must develop suddenly and increase in intensity within ten minutes of the beginning of the first symptom noticed in the attack. The panic attack must not be attributable to some known organic factor, e.g. amphetamine or caffeine, intoxication, hyperthyroidism. The efficacy of alprazolam in conditions where the above criteria are not met has not been established. The risk versus benefits of alprazolam use in milder disorders, which do not meet the above criteria, has not been evaluated. Although current evidence supports the long-term clinical effectiveness of alprazolam in panic disorder, the continuing use of alprazolam needs to be weighed against the difficulties that can occur with dependence and discontinuation. The results of a long-term study in patients taking alprazolam (ie. beyond three months) suggest that many patients continue to benefit from alprazolam therapy and that alprazolam efficacy is maintained for up to eight months. The physician should periodically reassess the usefulness of the drug for each patient. A comparative study of alprazolam and placebo in the treatment of panic attacks in patients with panic disorder involved 543 patients over an eight week period. Alprazolam was significantly more effective than placebo in reducing the total number of panic attacks (p < 0.0001); at week 4, 46.8% of alprazolam patients had achieved zero total panic attacks when compared to 27.1% of placebo patients. Panic disorders are often severe, chronic illnesses that cause a high level of work and social disability, increased substance abuse and potentially increased morbidity and mortality. Psychological and social factors are important in the pathogenesis of panic attacks, either acting alone or in combination with biological factors. Prolonged pharmacological therapy may be used as an adjunct to psychosocial therapy in the treatment of patients with panic disorders.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, uncoated
- Oral
- 9mm x 5.5mm oval, white tablet debossed AL/0.25 on one side, G on the reverse.
Images
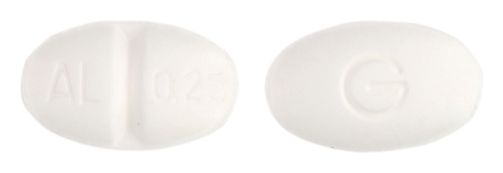

Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 25 degrees Celsius
- Shelf lifetime is 3 Years.
Do I need a prescription ?
(ARTG)
These medicines require authorisation for prescription from your doctor. It is Schedule 8 : Controlled Drug.
- 10 Tablets pack
- 50 tablets pack
Over 65 ?
(AHT)
This medicine contains the active ingredients:
If you are over 65 years of age, there may be specific risks and recommendations for use of this medicine. Please discuss your individual circumstances with your pharmacist, doctor or health professional. For more information read our page on medication safety for older people.
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient alprazolam
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: KALMA 0.25 alprazolam 0.25mg tablet blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
Listen to the audio transcription of the CMI leaflet.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
For short-term symptomatic treatment of anxiety including treatment of anxious patients with some symptoms of depression. Alprazolam is not recommended as primary therapy in patients with depression. For the treatment of panic disorder with or without some phobic avoidance, and for blocking or attenuation of panic attacks and phobias in patients who have agoraphobia with panic attacks.INDICATIONS AS AT 17 DECEMBER 2001 : Anxiety. Short-term symptomatic treatment of anxiety including treatment of anxious patients with some symptoms of depression. Panic disorder. The treatment of panic disorder with or without some phobic avoidance, and for blocking or attenuation of panic attacks and phobias in patients who have agoraphobia with panic attacks. The diagnostic criteria for panic disorder in DSM-III-R are as follows: The panic attacks (discrete periods of intense fear or discomfort), at least initially, are unexpected. Later in the course of this disturbance, certain situations (e.g. driving a car or being in a crowded place) may become associated with having a panic attack. These panic attacks are not triggered by situations in which the person is the focus of others' attention (as in social phobia). The diagnosis requires four such attacks within a four week period, or one or more attacks followed by at least a month of persistent fear of having another attack. The panic attacks must be characterised by at least four of the following symptoms: dyspnoea or smothering sensations; dizziness, unsteady feelings or faintness; palpitations or tachycardia; trembling or shaking; sweating; choking; nausea or abdominal distress; depersonalisation or derealisation; paraesthesiae; flushes (hot flashes) or chills; chest pain or discomfort; fear of dying; fear of going crazy or of doing something uncontrolled. Note. Attacks involving four or more symptoms are panic attacks; attacks involving fewer than four are limited symptom attacks. At least some of the panic attack symptoms must develop suddenly and increase in intensity within ten minutes of the beginning of the first symptom noticed in the attack. The panic attack must not be attributable to some known organic factor, e.g. amphetamine or caffeine, intoxication, hyperthyroidism. The efficacy of alprazolam in conditions where the above criteria are not met has not been established. The risk versus benefits of alprazolam use in milder disorders, which do not meet the above criteria, has not been evaluated. Although current evidence supports the long-term clinical effectiveness of alprazolam in panic disorder, the continuing use of alprazolam needs to be weighed against the difficulties that can occur with dependence and discontinuation. The results of a long-term study in patients taking alprazolam (ie. beyond three months) suggest that many patients continue to benefit from alprazolam therapy and that alprazolam efficacy is maintained for up to eight months. The physician should periodically reassess the usefulness of the drug for each patient. A comparative study of alprazolam and placebo in the treatment of panic attacks in patients with panic disorder involved 543 patients over an eight week period. Alprazolam was significantly more effective than placebo in reducing the total number of panic attacks (p < 0.0001); at week 4, 46.8% of alprazolam patients had achieved zero total panic attacks when compared to 27.1% of placebo patients. Panic disorders are often severe, chronic illnesses that cause a high level of work and social disability, increased substance abuse and potentially increased morbidity and mortality. Psychological and social factors are important in the pathogenesis of panic attacks, either acting alone or in combination with biological factors. Prolonged pharmacological therapy may be used as an adjunct to psychosocial therapy in the treatment of patients with panic disorders.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, uncoated
- Oral
- 9mm x 5.5mm oval, white tablet debossed AL/0.25 on one side, G on the reverse.
Images
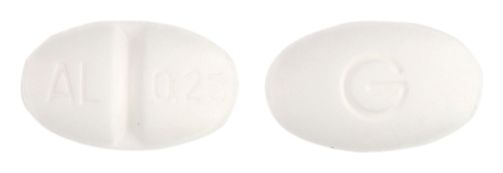

Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 25 degrees Celsius
- Shelf lifetime is 2 Years.
Do I need a prescription ?
(ARTG)
These medicines require authorisation for prescription from your doctor. It is Schedule 8 : Controlled Drug.
- 10 Tablets pack
- 50 tablets pack
Over 65 ?
(AHT)
This medicine contains the active ingredients:
If you are over 65 years of age, there may be specific risks and recommendations for use of this medicine. Please discuss your individual circumstances with your pharmacist, doctor or health professional. For more information read our page on medication safety for older people.
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient alprazolam
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: KALMA 0.5 alprazolam 0.5mg tablet bottleWhat this medicine is used for
(ARTG)
For short-term symptomatic treatment of anxiety including treatment of anxious patients with some symptoms of depression. Alprazolam is not recommended as primary therapy in patients with depression. For the treatment of panic disorder with or without some phobic avoidance, and for blocking or attenuation of panic attacks and phobias in patients who have agoraphobia with panic attacks. INDICATIONS AS AT 17 DECEMBER 2001 : Anxiety. Short-term symptomatic treatment of anxiety including treatment of anxious patients with some symptoms of depression. Panic disorder. The treatment of panic disorder with or without some phobic avoidance, and for blocking or attenuation of panic attacks and phobias in patients who have agoraphobia with panic attacks. The diagnostic criteria for panic disorder in DSM-III-R are as follows: The panic attacks (discrete periods of intense fear or discomfort), at least initially, are unexpected. Later in the course of this disturbance, certain situations (e.g. driving a car or being in a crowded place) may become associated with having a panic attack. These panic attacks are not triggered by situations in which the person is the focus of others' attention (as in social phobia). The diagnosis requires four such attacks within a four week period, or one or more attacks followed by at least a month of persistent fear of having another attack. The panic attacks must be characterised by at least four of the following symptoms: dyspnoea or smothering sensations; dizziness, unsteady feelings or faintness; palpitations or tachycardia; trembling or shaking; sweating; choking; nausea or abdominal distress; depersonalisation or derealisation; paraesthesiae; flushes (hot flashes) or chills; chest pain or discomfort; fear of dying; fear of going crazy or of doing something uncontrolled. Note. Attacks involving four or more symptoms are panic attacks; attacks involving fewer than four are limited symptom attacks. At least some of the panic attack symptoms must develop suddenly and increase in intensity within ten minutes of the beginning of the first symptom noticed in the attack. The panic attack must not be attributable to some known organic factor, e.g. amphetamine or caffeine, intoxication, hyperthyroidism. The efficacy of alprazolam in conditions where the above criteria are not met has not been established. The risk versus benefits of alprazolam use in milder disorders, which do not meet the above criteria, has not been evaluated. Although current evidence supports the long-term clinical effectiveness of alprazolam in panic disorder, the continuing use of alprazolam needs to be weighed against the difficulties that can occur with dependence and discontinuation. The results of a long-term study in patients taking alprazolam (ie. beyond three months) suggest that many patients continue to benefit from alprazolam therapy and that alprazolam efficacy is maintained for up to eight months. The physician should periodically reassess the usefulness of the drug for each patient. A comparative study of alprazolam and placebo in the treatment of panic attacks in patients with panic disorder involved 543 patients over an eight week period. Alprazolam was significantly more effective than placebo in reducing the total number of panic attacks (p < 0.0001); at week 4, 46.8% of alprazolam patients had achieved zero total panic attacks when compared to 27.1% of placebo patients. Panic disorders are often severe, chronic illnesses that cause a high level of work and social disability, increased substance abuse and potentially increased morbidity and mortality. Psychological and social factors are important in the pathogenesis of panic attacks, either acting alone or in combination with biological factors. Prolonged pharmacological therapy may be used as an adjunct to psychosocial therapy in the treatment of patients with panic disorders.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, uncoated
- Oral
- 9mm x 5.5mm oval, pale pink tablet debossed AL/0.5 on one side, G on the reverse.
Images
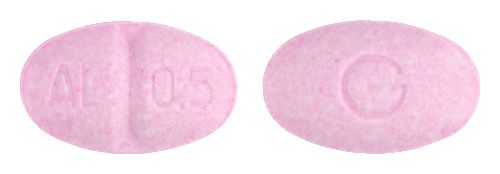

Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 25 degrees Celsius
- Shelf lifetime is 3 Years.
Do I need a prescription ?
(ARTG)
These medicines require authorisation for prescription from your doctor. It is Schedule 8 : Controlled Drug.
- 10 Tablets pack
- 50 tablets pack
Over 65 ?
(AHT)
This medicine contains the active ingredients:
If you are over 65 years of age, there may be specific risks and recommendations for use of this medicine. Please discuss your individual circumstances with your pharmacist, doctor or health professional. For more information read our page on medication safety for older people.
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient alprazolam
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: KALMA 0.5 alprazolam 0.5mg tablet blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
Listen to the audio transcription of the CMI leaflet.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
For short-term symptomatic treatment of anxiety including treatment of anxious patients with some symptoms of depression. Alprazolam is not recommended as primary therapy in patients with depression. For the treatment of panic disorder with or without some phobic avoidance, and for blocking or attenuation of panic attacks and phobias in patients who have agoraphobia with panic attacks. INDICATIONS AS AT 17 DECEMBER 2001 : Anxiety. Short-term symptomatic treatment of anxiety including treatment of anxious patients with some symptoms of depression. Panic disorder. The treatment of panic disorder with or without some phobic avoidance, and for blocking or attenuation of panic attacks and phobias in patients who have agoraphobia with panic attacks. The diagnostic criteria for panic disorder in DSM-III-R are as follows: The panic attacks (discrete periods of intense fear or discomfort), at least initially, are unexpected. Later in the course of this disturbance, certain situations (e.g. driving a car or being in a crowded place) may become associated with having a panic attack. These panic attacks are not triggered by situations in which the person is the focus of others' attention (as in social phobia). The diagnosis requires four such attacks within a four week period, or one or more attacks followed by at least a month of persistent fear of having another attack. The panic attacks must be characterised by at least four of the following symptoms: dyspnoea or smothering sensations; dizziness, unsteady feelings or faintness; palpitations or tachycardia; trembling or shaking; sweating; choking; nausea or abdominal distress; depersonalisation or derealisation; paraesthesiae; flushes (hot flashes) or chills; chest pain or discomfort; fear of dying; fear of going crazy or of doing something uncontrolled. Note. Attacks involving four or more symptoms are panic attacks; attacks involving fewer than four are limited symptom attacks. At least some of the panic attack symptoms must develop suddenly and increase in intensity within ten minutes of the beginning of the first symptom noticed in the attack. The panic attack must not be attributable to some known organic factor, e.g. amphetamine or caffeine, intoxication, hyperthyroidism. The efficacy of alprazolam in conditions where the above criteria are not met has not been established. The risk versus benefits of alprazolam use in milder disorders, which do not meet the above criteria, has not been evaluated. Although current evidence supports the long-term clinical effectiveness of alprazolam in panic disorder, the continuing use of alprazolam needs to be weighed against the difficulties that can occur with dependence and discontinuation. The results of a long-term study in patients taking alprazolam (ie. beyond three months) suggest that many patients continue to benefit from alprazolam therapy and that alprazolam efficacy is maintained for up to eight months. The physician should periodically reassess the usefulness of the drug for each patient. A comparative study of alprazolam and placebo in the treatment of panic attacks in patients with panic disorder involved 543 patients over an eight week period. Alprazolam was significantly more effective than placebo in reducing the total number of panic attacks (p < 0.0001); at week 4, 46.8% of alprazolam patients had achieved zero total panic attacks when compared to 27.1% of placebo patients. Panic disorders are often severe, chronic illnesses that cause a high level of work and social disability, increased substance abuse and potentially increased morbidity and mortality. Psychological and social factors are important in the pathogenesis of panic attacks, either acting alone or in combination with biological factors. Prolonged pharmacological therapy may be used as an adjunct to psychosocial therapy in the treatment of patients with panic disorders.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, uncoated
- Oral
- 9mm x 5.5mm oval, pale pink tablet debossed AL/0.5 on one side, G on the reverse.
Images
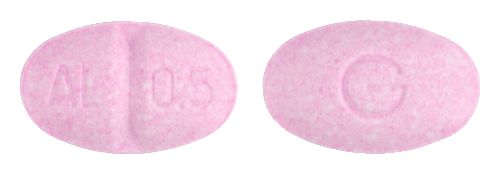

Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 25 degrees Celsius
- Shelf lifetime is 2 Years.
Do I need a prescription ?
(ARTG)
These medicines require authorisation for prescription from your doctor. It is Schedule 8 : Controlled Drug.
- 10 Tablets pack
- 50 tablets pack
Over 65 ?
(AHT)
This medicine contains the active ingredients:
If you are over 65 years of age, there may be specific risks and recommendations for use of this medicine. Please discuss your individual circumstances with your pharmacist, doctor or health professional. For more information read our page on medication safety for older people.
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient alprazolam
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: KALMA 1 alprazolam 1mg tablet bottleWhat this medicine is used for
(ARTG)
For short-term symptomatic treatment of anxiety including treatment of anxious patients with some symptoms of depression. Alprazolam is not recommended as primary therapy in patients with depression. For the treatment of panic disorder with or without some phobic avoidance, and for blocking or attenuation of panic attacks and phobias in patients who have agoraphobia with panic attacks. INDICATIONS AS AT 17 DECEMBER 2001 : Anxiety. Short-term symptomatic treatment of anxiety including treatment of anxious patients with some symptoms of depression. Panic disorder. The treatment of panic disorder with or without some phobic avoidance, and for blocking or attenuation of panic attacks and phobias in patients who have agoraphobia with panic attacks. The diagnostic criteria for panic disorder in DSM-III-R are as follows: The panic attacks (discrete periods of intense fear or discomfort), at least initially, are unexpected. Later in the course of this disturbance, certain situations (e.g. driving a car or being in a crowded place) may become associated with having a panic attack. These panic attacks are not triggered by situations in which the person is the focus of others' attention (as in social phobia). The diagnosis requires four such attacks within a four week period, or one or more attacks followed by at least a month of persistent fear of having another attack. The panic attacks must be characterised by at least four of the following symptoms: dyspnoea or smothering sensations; dizziness, unsteady feelings or faintness; palpitations or tachycardia; trembling or shaking; sweating; choking; nausea or abdominal distress; depersonalisation or derealisation; paraesthesiae; flushes (hot flashes) or chills; chest pain or discomfort; fear of dying; fear of going crazy or of doing something uncontrolled. Note. Attacks involving four or more symptoms are panic attacks; attacks involving fewer than four are limited symptom attacks. At least some of the panic attack symptoms must develop suddenly and increase in intensity within ten minutes of the beginning of the first symptom noticed in the attack. The panic attack must not be attributable to some known organic factor, e.g. amphetamine or caffeine, intoxication, hyperthyroidism. The efficacy of alprazolam in conditions where the above criteria are not met has not been established. The risk versus benefits of alprazolam use in milder disorders, which do not meet the above criteria, has not been evaluated. Although current evidence supports the long-term clinical effectiveness of alprazolam in panic disorder, the continuing use of alprazolam needs to be weighed against the difficulties that can occur with dependence and discontinuation. The results of a long-term study in patients taking alprazolam (ie. beyond three months) suggest that many patients continue to benefit from alprazolam therapy and that alprazolam efficacy is maintained for up to eight months. The physician should periodically reassess the usefulness of the drug for each patient. A comparative study of alprazolam and placebo in the treatment of panic attacks in patients with panic disorder involved 543 patients over an eight week period. Alprazolam was significantly more effective than placebo in reducing the total number of panic attacks (p < 0.0001); at week 4, 46.8% of alprazolam patients had achieved zero total panic attacks when compared to 27.1% of placebo patients. Panic disorders are often severe, chronic illnesses that cause a high level of work and social disability, increased substance abuse and potentially increased morbidity and mortality. Psychological and social factors are important in the pathogenesis of panic attacks, either acting alone or in combination with biological factors. Prolonged pharmacological therapy may be used as an adjunct to psychosocial therapy in the treatment of patients with panic disorders.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, uncoated
- Oral
- 9mm x 5.5mm oval, pale blue tablet debossed AL/1.0 on one side, G on the reverse.
Images
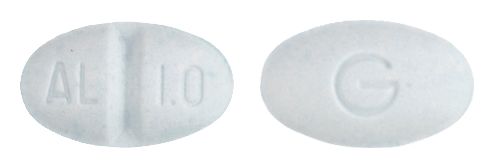

Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 25 degrees Celsius
- Shelf lifetime is 3 Years.
Do I need a prescription ?
(ARTG)
These medicines require authorisation for prescription from your doctor. It is Schedule 8 : Controlled Drug.
- 10 tablets_(1mg) pack
- 50 tablets pack
Over 65 ?
(AHT)
This medicine contains the active ingredients:
If you are over 65 years of age, there may be specific risks and recommendations for use of this medicine. Please discuss your individual circumstances with your pharmacist, doctor or health professional. For more information read our page on medication safety for older people.
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient alprazolam
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: KALMA 1 alprazolam 1mg tablet blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
Listen to the audio transcription of the CMI leaflet.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
For short-term symptomatic treatment of anxiety including treatment of anxious patients with some symptoms of depression. Alprazolam is not recommended as primary therapy in patients with depression. For the treatment of panic disorder with or without some phobic avoidance, and for blocking or attenuation of panic attacks and phobias in patients who have agoraphobia with panic attacks. INDICATIONS AS AT 17 DECEMBER 2001 : Anxiety. Short-term symptomatic treatment of anxiety including treatment of anxious patients with some symptoms of depression. Panic disorder. The treatment of panic disorder with or without some phobic avoidance, and for blocking or attenuation of panic attacks and phobias in patients who have agoraphobia with panic attacks. The diagnostic criteria for panic disorder in DSM-III-R are as follows: The panic attacks (discrete periods of intense fear or discomfort), at least initially, are unexpected. Later in the course of this disturbance, certain situations (e.g. driving a car or being in a crowded place) may become associated with having a panic attack. These panic attacks are not triggered by situations in which the person is the focus of others' attention (as in social phobia). The diagnosis requires four such attacks within a four week period, or one or more attacks followed by at least a month of persistent fear of having another attack. The panic attacks must be characterised by at least four of the following symptoms: dyspnoea or smothering sensations; dizziness, unsteady feelings or faintness; palpitations or tachycardia; trembling or shaking; sweating; choking; nausea or abdominal distress; depersonalisation or derealisation; paraesthesiae; flushes (hot flashes) or chills; chest pain or discomfort; fear of dying; fear of going crazy or of doing something uncontrolled. Note. Attacks involving four or more symptoms are panic attacks; attacks involving fewer than four are limited symptom attacks. At least some of the panic attack symptoms must develop suddenly and increase in intensity within ten minutes of the beginning of the first symptom noticed in the attack. The panic attack must not be attributable to some known organic factor, e.g. amphetamine or caffeine, intoxication, hyperthyroidism. The efficacy of alprazolam in conditions where the above criteria are not met has not been established. The risk versus benefits of alprazolam use in milder disorders, which do not meet the above criteria, has not been evaluated. Although current evidence supports the long-term clinical effectiveness of alprazolam in panic disorder, the continuing use of alprazolam needs to be weighed against the difficulties that can occur with dependence and discontinuation. The results of a long-term study in patients taking alprazolam (ie. beyond three months) suggest that many patients continue to benefit from alprazolam therapy and that alprazolam efficacy is maintained for up to eight months. The physician should periodically reassess the usefulness of the drug for each patient. A comparative study of alprazolam and placebo in the treatment of panic attacks in patients with panic disorder involved 543 patients over an eight week period. Alprazolam was significantly more effective than placebo in reducing the total number of panic attacks (p < 0.0001); at week 4, 46.8% of alprazolam patients had achieved zero total panic attacks when compared to 27.1% of placebo patients. Panic disorders are often severe, chronic illnesses that cause a high level of work and social disability, increased substance abuse and potentially increased morbidity and mortality. Psychological and social factors are important in the pathogenesis of panic attacks, either acting alone or in combination with biological factors. Prolonged pharmacological therapy may be used as an adjunct to psychosocial therapy in the treatment of patients with panic disorders.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, uncoated
- Oral
- 9mm x 5.5mm oval, pale blue tablet debossed AL/1.0 on one side, G on the reverse.
Images
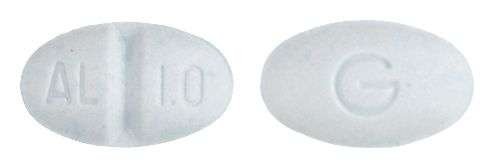

Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 25 degrees Celsius
- Shelf lifetime is 2 Years.
Do I need a prescription ?
(ARTG)
These medicines require authorisation for prescription from your doctor. It is Schedule 8 : Controlled Drug.
- 10 tablets_(1mg) pack
- 50 tablets pack
Over 65 ?
(AHT)
This medicine contains the active ingredients:
If you are over 65 years of age, there may be specific risks and recommendations for use of this medicine. Please discuss your individual circumstances with your pharmacist, doctor or health professional. For more information read our page on medication safety for older people.
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient alprazolam
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: KALMA 2 alprazolam 2mg tablet bottleWhat this medicine is used for
(ARTG)
Anxiety. Short-term symptomatic treatment of anxiety including treatment of anxious patients with some symptoms of depression. Panic disorder. The treatment of panic disorder with or without some phobic avoidance, and for blocking or attenuation of panic attacks and phobias in patients who have agoraphobia with panic attacks. The diagnostic criteria for panic disorder in DSM-III-R are as follows: The panic attacks (discrete periods of intense fear or discomfort), at least initially, are unexpected. Later in the course of this disturbance, certain situations (eg. driving a car or being in a crowded place) may become associated with having a panic attack. These panic attacks are not triggered by situations in which the person is the focus of others' attention (as in social phobia). The diagnosis requires four such attacks within a four week period, or one or more attacks followed by at least a month of persistent fear of having another attack. The panic attacks must be characterised by at least four of the following symptoms: dyspnoea or smothering sensations; dizziness, unsteady feelings or faintness; palpitations or tachycardia; trembling or shaking; sweating; choking; nausea or abdominal distress; depersonalisation or derealisation; paraesthesiae; flushes (hot flashes) or chills; chest pain or discomfort; fear of dying; fear of going crazy or of doing something uncontrolled. NOTE: Attacks involving four or more symptoms are panic attacks; attacks involving fewer than four are limited symptom attacks. At least some of the panic attack symptoms must develop suddenly and increase in intensity within ten minutes of the beginning of the first symptom noticed in the attack. The panic attack must not be attributable to some known organic factor, eg. amphetamine or caffeine, intoxication, hyper-thyroidism. The efficacy of alprazolam in conditions where the above criteria are not met has not been established. The risk versus benefits of alprazolam use in milder disorders, which do not meet the above criteria, has not been evaluated. Although current evidence supports the long-term clinical effectiveness of alprazolam in panic disorder, the continuing use of alprazolam needs to be weighed against the difficulties that can occur with dependence and discontinuation. The results of a long-term study in patients taking alprazolam (ie. beyond three months) suggest that many patients continue to benefit from alprazolam therapy and that alprazolam efficacy is maintained for up to eight months. The physician should periodically reassess the usefulness of the drug for each patient. A comparative study of alprazolam and placebo in the treatment of panic attacks in patients with panic disorder involved 543 patients over an eight week period. Alprazolam was significantly more effective than placebo in reducing the total number of panic attacks (p<0.0001); at week 4, 46.8% of alprazolam patients had achieved zero total panic attacks when compared to 27.1% of placebo patients. Panic disorders are often severe, chronic illnesses that cause a high level of work and social disability, increased substance abuse and potentially increased morbidity and mortality. Psychological and social factors are important in the pathogenesis of panic attacks, either acting alone or in combination with biological factors. Prolonged pharmacological therapy may be used as an adjunct to psychosocial therapy in the treatment of patients with panic disorders.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, uncoated
- Oral
- 9.5 mm x 9.0 mm white oval bevel edged quadrisect tablet marked "A" in the upper left quadrant "L" in the upper right quadrant "G" in the lower left quadrant, "2" in the lower right quadrant on one side, plain quadrisect on the other side.
Images


Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 25 degrees Celsius
- Shelf lifetime is 3 Years.
Do I need a prescription ?
(ARTG)
These medicines require authorisation for prescription from your doctor. It is Schedule 8 : Controlled Drug.
- 50 tablets pack
Over 65 ?
(AHT)
This medicine contains the active ingredients:
If you are over 65 years of age, there may be specific risks and recommendations for use of this medicine. Please discuss your individual circumstances with your pharmacist, doctor or health professional. For more information read our page on medication safety for older people.
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient alprazolam
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: KALMA 0.25 alprazolam 0.25mg tablet bottleWhat this medicine is used for
(ARTG)
For short-term symptomatic treatment of anxiety including treatment of anxious patients with some symptoms of depression. Alprazolam is not recommended as primary therapy in patients with depression. For the treatment of panic disorder with or without some phobic avoidance, and for blocking or attenuation of panic attacks and phobias in patients who have agoraphobia with panic attacks.INDICATIONS AS AT 17 DECEMBER 2001 : Anxiety. Short-term symptomatic treatment of anxiety including treatment of anxious patients with some symptoms of depression. Panic disorder. The treatment of panic disorder with or without some phobic avoidance, and for blocking or attenuation of panic attacks and phobias in patients who have agoraphobia with panic attacks. The diagnostic criteria for panic disorder in DSM-III-R are as follows: The panic attacks (discrete periods of intense fear or discomfort), at least initially, are unexpected. Later in the course of this disturbance, certain situations (e.g. driving a car or being in a crowded place) may become associated with having a panic attack. These panic attacks are not triggered by situations in which the person is the focus of others' attention (as in social phobia). The diagnosis requires four such attacks within a four week period, or one or more attacks followed by at least a month of persistent fear of having another attack. The panic attacks must be characterised by at least four of the following symptoms: dyspnoea or smothering sensations; dizziness, unsteady feelings or faintness; palpitations or tachycardia; trembling or shaking; sweating; choking; nausea or abdominal distress; depersonalisation or derealisation; paraesthesiae; flushes (hot flashes) or chills; chest pain or discomfort; fear of dying; fear of going crazy or of doing something uncontrolled. Note. Attacks involving four or more symptoms are panic attacks; attacks involving fewer than four are limited symptom attacks. At least some of the panic attack symptoms must develop suddenly and increase in intensity within ten minutes of the beginning of the first symptom noticed in the attack. The panic attack must not be attributable to some known organic factor, e.g. amphetamine or caffeine, intoxication, hyperthyroidism. The efficacy of alprazolam in conditions where the above criteria are not met has not been established. The risk versus benefits of alprazolam use in milder disorders, which do not meet the above criteria, has not been evaluated. Although current evidence supports the long-term clinical effectiveness of alprazolam in panic disorder, the continuing use of alprazolam needs to be weighed against the difficulties that can occur with dependence and discontinuation. The results of a long-term study in patients taking alprazolam (ie. beyond three months) suggest that many patients continue to benefit from alprazolam therapy and that alprazolam efficacy is maintained for up to eight months. The physician should periodically reassess the usefulness of the drug for each patient. A comparative study of alprazolam and placebo in the treatment of panic attacks in patients with panic disorder involved 543 patients over an eight week period. Alprazolam was significantly more effective than placebo in reducing the total number of panic attacks (p < 0.0001); at week 4, 46.8% of alprazolam patients had achieved zero total panic attacks when compared to 27.1% of placebo patients. Panic disorders are often severe, chronic illnesses that cause a high level of work and social disability, increased substance abuse and potentially increased morbidity and mortality. Psychological and social factors are important in the pathogenesis of panic attacks, either acting alone or in combination with biological factors. Prolonged pharmacological therapy may be used as an adjunct to psychosocial therapy in the treatment of patients with panic disorders.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, uncoated
- Oral
- 9mm x 5.5mm oval, white tablet debossed AL/0.25 on one side, G on the reverse.
Images


Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 25 degrees Celsius
- Shelf lifetime is 3 Years.
Do I need a prescription ?
(ARTG)
These medicines require authorisation for prescription from your doctor. It is Schedule 8 : Controlled Drug.
- 10 Tablets pack
- 50 tablets pack
Is this medicine subsidised ?
(PBS)
This medicine was verified as being available on the PBS (Pharmaceutical Benefits Scheme) on October, 1 2025. To learn more about this subsidy, visit the Pharmaceutical Benefits Scheme (PBS) website.
Over 65 ?
(AHT)
This medicine contains the active ingredients:
If you are over 65 years of age, there may be specific risks and recommendations for use of this medicine. Please discuss your individual circumstances with your pharmacist, doctor or health professional. For more information read our page on medication safety for older people.
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient alprazolam
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: KALMA 0.5 alprazolam 0.5mg tablet bottleWhat this medicine is used for
(ARTG)
For short-term symptomatic treatment of anxiety including treatment of anxious patients with some symptoms of depression. Alprazolam is not recommended as primary therapy in patients with depression. For the treatment of panic disorder with or without some phobic avoidance, and for blocking or attenuation of panic attacks and phobias in patients who have agoraphobia with panic attacks. INDICATIONS AS AT 17 DECEMBER 2001 : Anxiety. Short-term symptomatic treatment of anxiety including treatment of anxious patients with some symptoms of depression. Panic disorder. The treatment of panic disorder with or without some phobic avoidance, and for blocking or attenuation of panic attacks and phobias in patients who have agoraphobia with panic attacks. The diagnostic criteria for panic disorder in DSM-III-R are as follows: The panic attacks (discrete periods of intense fear or discomfort), at least initially, are unexpected. Later in the course of this disturbance, certain situations (e.g. driving a car or being in a crowded place) may become associated with having a panic attack. These panic attacks are not triggered by situations in which the person is the focus of others' attention (as in social phobia). The diagnosis requires four such attacks within a four week period, or one or more attacks followed by at least a month of persistent fear of having another attack. The panic attacks must be characterised by at least four of the following symptoms: dyspnoea or smothering sensations; dizziness, unsteady feelings or faintness; palpitations or tachycardia; trembling or shaking; sweating; choking; nausea or abdominal distress; depersonalisation or derealisation; paraesthesiae; flushes (hot flashes) or chills; chest pain or discomfort; fear of dying; fear of going crazy or of doing something uncontrolled. Note. Attacks involving four or more symptoms are panic attacks; attacks involving fewer than four are limited symptom attacks. At least some of the panic attack symptoms must develop suddenly and increase in intensity within ten minutes of the beginning of the first symptom noticed in the attack. The panic attack must not be attributable to some known organic factor, e.g. amphetamine or caffeine, intoxication, hyperthyroidism. The efficacy of alprazolam in conditions where the above criteria are not met has not been established. The risk versus benefits of alprazolam use in milder disorders, which do not meet the above criteria, has not been evaluated. Although current evidence supports the long-term clinical effectiveness of alprazolam in panic disorder, the continuing use of alprazolam needs to be weighed against the difficulties that can occur with dependence and discontinuation. The results of a long-term study in patients taking alprazolam (ie. beyond three months) suggest that many patients continue to benefit from alprazolam therapy and that alprazolam efficacy is maintained for up to eight months. The physician should periodically reassess the usefulness of the drug for each patient. A comparative study of alprazolam and placebo in the treatment of panic attacks in patients with panic disorder involved 543 patients over an eight week period. Alprazolam was significantly more effective than placebo in reducing the total number of panic attacks (p < 0.0001); at week 4, 46.8% of alprazolam patients had achieved zero total panic attacks when compared to 27.1% of placebo patients. Panic disorders are often severe, chronic illnesses that cause a high level of work and social disability, increased substance abuse and potentially increased morbidity and mortality. Psychological and social factors are important in the pathogenesis of panic attacks, either acting alone or in combination with biological factors. Prolonged pharmacological therapy may be used as an adjunct to psychosocial therapy in the treatment of patients with panic disorders.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, uncoated
- Oral
- 9mm x 5.5mm oval, pale pink tablet debossed AL/0.5 on one side, G on the reverse.
Images


Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 25 degrees Celsius
- Shelf lifetime is 3 Years.
Do I need a prescription ?
(ARTG)
These medicines require authorisation for prescription from your doctor. It is Schedule 8 : Controlled Drug.
- 10 Tablets pack
- 50 tablets pack
Is this medicine subsidised ?
(PBS)
This medicine was verified as being available on the PBS (Pharmaceutical Benefits Scheme) on October, 1 2025. To learn more about this subsidy, visit the Pharmaceutical Benefits Scheme (PBS) website.
Over 65 ?
(AHT)
This medicine contains the active ingredients:
If you are over 65 years of age, there may be specific risks and recommendations for use of this medicine. Please discuss your individual circumstances with your pharmacist, doctor or health professional. For more information read our page on medication safety for older people.
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient alprazolam
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: KALMA 1 alprazolam 1mg tablet bottleWhat this medicine is used for
(ARTG)
For short-term symptomatic treatment of anxiety including treatment of anxious patients with some symptoms of depression. Alprazolam is not recommended as primary therapy in patients with depression. For the treatment of panic disorder with or without some phobic avoidance, and for blocking or attenuation of panic attacks and phobias in patients who have agoraphobia with panic attacks. INDICATIONS AS AT 17 DECEMBER 2001 : Anxiety. Short-term symptomatic treatment of anxiety including treatment of anxious patients with some symptoms of depression. Panic disorder. The treatment of panic disorder with or without some phobic avoidance, and for blocking or attenuation of panic attacks and phobias in patients who have agoraphobia with panic attacks. The diagnostic criteria for panic disorder in DSM-III-R are as follows: The panic attacks (discrete periods of intense fear or discomfort), at least initially, are unexpected. Later in the course of this disturbance, certain situations (e.g. driving a car or being in a crowded place) may become associated with having a panic attack. These panic attacks are not triggered by situations in which the person is the focus of others' attention (as in social phobia). The diagnosis requires four such attacks within a four week period, or one or more attacks followed by at least a month of persistent fear of having another attack. The panic attacks must be characterised by at least four of the following symptoms: dyspnoea or smothering sensations; dizziness, unsteady feelings or faintness; palpitations or tachycardia; trembling or shaking; sweating; choking; nausea or abdominal distress; depersonalisation or derealisation; paraesthesiae; flushes (hot flashes) or chills; chest pain or discomfort; fear of dying; fear of going crazy or of doing something uncontrolled. Note. Attacks involving four or more symptoms are panic attacks; attacks involving fewer than four are limited symptom attacks. At least some of the panic attack symptoms must develop suddenly and increase in intensity within ten minutes of the beginning of the first symptom noticed in the attack. The panic attack must not be attributable to some known organic factor, e.g. amphetamine or caffeine, intoxication, hyperthyroidism. The efficacy of alprazolam in conditions where the above criteria are not met has not been established. The risk versus benefits of alprazolam use in milder disorders, which do not meet the above criteria, has not been evaluated. Although current evidence supports the long-term clinical effectiveness of alprazolam in panic disorder, the continuing use of alprazolam needs to be weighed against the difficulties that can occur with dependence and discontinuation. The results of a long-term study in patients taking alprazolam (ie. beyond three months) suggest that many patients continue to benefit from alprazolam therapy and that alprazolam efficacy is maintained for up to eight months. The physician should periodically reassess the usefulness of the drug for each patient. A comparative study of alprazolam and placebo in the treatment of panic attacks in patients with panic disorder involved 543 patients over an eight week period. Alprazolam was significantly more effective than placebo in reducing the total number of panic attacks (p < 0.0001); at week 4, 46.8% of alprazolam patients had achieved zero total panic attacks when compared to 27.1% of placebo patients. Panic disorders are often severe, chronic illnesses that cause a high level of work and social disability, increased substance abuse and potentially increased morbidity and mortality. Psychological and social factors are important in the pathogenesis of panic attacks, either acting alone or in combination with biological factors. Prolonged pharmacological therapy may be used as an adjunct to psychosocial therapy in the treatment of patients with panic disorders.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, uncoated
- Oral
- 9mm x 5.5mm oval, pale blue tablet debossed AL/1.0 on one side, G on the reverse.
Images


Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 25 degrees Celsius
- Shelf lifetime is 3 Years.
Do I need a prescription ?
(ARTG)
These medicines require authorisation for prescription from your doctor. It is Schedule 8 : Controlled Drug.
- 10 tablets_(1mg) pack
- 50 tablets pack
Is this medicine subsidised ?
(PBS)
This medicine was verified as being available on the PBS (Pharmaceutical Benefits Scheme) on October, 1 2025. To learn more about this subsidy, visit the Pharmaceutical Benefits Scheme (PBS) website.
Over 65 ?
(AHT)
This medicine contains the active ingredients:
If you are over 65 years of age, there may be specific risks and recommendations for use of this medicine. Please discuss your individual circumstances with your pharmacist, doctor or health professional. For more information read our page on medication safety for older people.
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient alprazolam
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: KALMA 0.25 alprazolam 0.25mg tablet blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
Listen to the audio transcription of the CMI leaflet.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
For short-term symptomatic treatment of anxiety including treatment of anxious patients with some symptoms of depression. Alprazolam is not recommended as primary therapy in patients with depression. For the treatment of panic disorder with or without some phobic avoidance, and for blocking or attenuation of panic attacks and phobias in patients who have agoraphobia with panic attacks.INDICATIONS AS AT 17 DECEMBER 2001 : Anxiety. Short-term symptomatic treatment of anxiety including treatment of anxious patients with some symptoms of depression. Panic disorder. The treatment of panic disorder with or without some phobic avoidance, and for blocking or attenuation of panic attacks and phobias in patients who have agoraphobia with panic attacks. The diagnostic criteria for panic disorder in DSM-III-R are as follows: The panic attacks (discrete periods of intense fear or discomfort), at least initially, are unexpected. Later in the course of this disturbance, certain situations (e.g. driving a car or being in a crowded place) may become associated with having a panic attack. These panic attacks are not triggered by situations in which the person is the focus of others' attention (as in social phobia). The diagnosis requires four such attacks within a four week period, or one or more attacks followed by at least a month of persistent fear of having another attack. The panic attacks must be characterised by at least four of the following symptoms: dyspnoea or smothering sensations; dizziness, unsteady feelings or faintness; palpitations or tachycardia; trembling or shaking; sweating; choking; nausea or abdominal distress; depersonalisation or derealisation; paraesthesiae; flushes (hot flashes) or chills; chest pain or discomfort; fear of dying; fear of going crazy or of doing something uncontrolled. Note. Attacks involving four or more symptoms are panic attacks; attacks involving fewer than four are limited symptom attacks. At least some of the panic attack symptoms must develop suddenly and increase in intensity within ten minutes of the beginning of the first symptom noticed in the attack. The panic attack must not be attributable to some known organic factor, e.g. amphetamine or caffeine, intoxication, hyperthyroidism. The efficacy of alprazolam in conditions where the above criteria are not met has not been established. The risk versus benefits of alprazolam use in milder disorders, which do not meet the above criteria, has not been evaluated. Although current evidence supports the long-term clinical effectiveness of alprazolam in panic disorder, the continuing use of alprazolam needs to be weighed against the difficulties that can occur with dependence and discontinuation. The results of a long-term study in patients taking alprazolam (ie. beyond three months) suggest that many patients continue to benefit from alprazolam therapy and that alprazolam efficacy is maintained for up to eight months. The physician should periodically reassess the usefulness of the drug for each patient. A comparative study of alprazolam and placebo in the treatment of panic attacks in patients with panic disorder involved 543 patients over an eight week period. Alprazolam was significantly more effective than placebo in reducing the total number of panic attacks (p < 0.0001); at week 4, 46.8% of alprazolam patients had achieved zero total panic attacks when compared to 27.1% of placebo patients. Panic disorders are often severe, chronic illnesses that cause a high level of work and social disability, increased substance abuse and potentially increased morbidity and mortality. Psychological and social factors are important in the pathogenesis of panic attacks, either acting alone or in combination with biological factors. Prolonged pharmacological therapy may be used as an adjunct to psychosocial therapy in the treatment of patients with panic disorders.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, uncoated
- Oral
- 9mm x 5.5mm oval, white tablet debossed AL/0.25 on one side, G on the reverse.
Images


Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 25 degrees Celsius
- Shelf lifetime is 2 Years.
Do I need a prescription ?
(ARTG)
These medicines require authorisation for prescription from your doctor. It is Schedule 8 : Controlled Drug.
- 10 Tablets pack
- 50 tablets pack
Is this medicine subsidised ?
(PBS)
This medicine was verified as being available on the PBS (Pharmaceutical Benefits Scheme) on October, 1 2025. To learn more about this subsidy, visit the Pharmaceutical Benefits Scheme (PBS) website.
Over 65 ?
(AHT)
This medicine contains the active ingredients:
If you are over 65 years of age, there may be specific risks and recommendations for use of this medicine. Please discuss your individual circumstances with your pharmacist, doctor or health professional. For more information read our page on medication safety for older people.
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient alprazolam
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: KALMA 2 alprazolam 2mg tablet blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
Listen to the audio transcription of the CMI leaflet.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Anxiety. Short-term symptomatic treatment of anxiety including treatment of anxious patients with some symptoms of depression. Panic disorder. The treatment of panic disorder with or without some phobic avoidance, and for blocking or attenuation of panic attacks and phobias in patients who have agoraphobia with panic attacks. The diagnostic criteria for panic disorder in DSM-III-R are as follows: The panic attacks (discrete periods of intense fear or discomfort), at least initially, are unexpected. Later in the course of this disturbance, certain situations (eg. driving a car or being in a crowded place) may become associated with having a panic attack. These panic attacks are not triggered by situations in which the person is the focus of others' attention (as in social phobia). The diagnosis requires four such attacks within a four week period, or one or more attacks followed by at least a month of persistent fear of having another attack. The panic attacks must be characterised by at least four of the following symptoms: dyspnoea or smothering sensations; dizziness, unsteady feelings or faintness; palpitations or tachycardia; trembling or shaking; sweating; choking; nausea or abdominal distress; depersonalisation or derealisation; paraesthesiae; flushes (hot flashes) or chills; chest pain or discomfort; fear of dying; fear of going crazy or of doing something uncontrolled. NOTE: Attacks involving four or more symptoms are panic attacks; attacks involving fewer than four are limited symptom attacks. At least some of the panic attack symptoms must develop suddenly and increase in intensity within ten minutes of the beginning of the first symptom noticed in the attack. The panic attack must not be attributable to some known organic factor, eg. amphetamine or caffeine, intoxication, hyper-thyroidism. The efficacy of alprazolam in conditions where the above criteria are not met has not been established. The risk versus benefits of alprazolam use in milder disorders, which do not meet the above criteria, has not been evaluated. Although current evidence supports the long-term clinical effectiveness of alprazolam in panic disorder, the continuing use of alprazolam needs to be weighed against the difficulties that can occur with dependence and discontinuation. The results of a long-term study in patients taking alprazolam (ie. beyond three months) suggest that many patients continue to benefit from alprazolam therapy and that alprazolam efficacy is maintained for up to eight months. The physician should periodically reassess the usefulness of the drug for each patient. A comparative study of alprazolam and placebo in the treatment of panic attacks in patients with panic disorder involved 543 patients over an eight week period. Alprazolam was significantly more effective than placebo in reducing the total number of panic attacks (p<0.0001); at week 4, 46.8% of alprazolam patients had achieved zero total panic attacks when compared to 27.1% of placebo patients. Panic disorders are often severe, chronic illnesses that cause a high level of work and social disability, increased substance abuse and potentially increased morbidity and mortality. Psychological and social factors are important in the pathogenesis of panic attacks, either acting alone or in combination with biological factors. Prolonged pharmacological therapy may be used as an adjunct to psychosocial therapy in the treatment of patients with panic disorders.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, uncoated
- Oral
- 9.5 mm x 9.0 mm white oval bevel edged quadrisect tablet marked "A" in the upper left quadrant "L" in the upper right quadrant "G" in the lower left quadrant, "2" in the lower right quadrant on one side, plain quadrisect on the other side.
Images
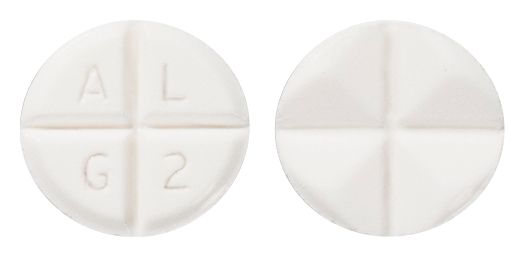

Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 25 degrees Celsius
- Shelf lifetime is 2 Years.
Do I need a prescription ?
(ARTG)
These medicines require authorisation for prescription from your doctor. It is Schedule 8 : Controlled Drug.
- 50 tablets pack
Over 65 ?
(AHT)
This medicine contains the active ingredients:
If you are over 65 years of age, there may be specific risks and recommendations for use of this medicine. Please discuss your individual circumstances with your pharmacist, doctor or health professional. For more information read our page on medication safety for older people.
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient alprazolam
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: KALMA 1 alprazolam 1mg tablet blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
Listen to the audio transcription of the CMI leaflet.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
For short-term symptomatic treatment of anxiety including treatment of anxious patients with some symptoms of depression. Alprazolam is not recommended as primary therapy in patients with depression. For the treatment of panic disorder with or without some phobic avoidance, and for blocking or attenuation of panic attacks and phobias in patients who have agoraphobia with panic attacks. INDICATIONS AS AT 17 DECEMBER 2001 : Anxiety. Short-term symptomatic treatment of anxiety including treatment of anxious patients with some symptoms of depression. Panic disorder. The treatment of panic disorder with or without some phobic avoidance, and for blocking or attenuation of panic attacks and phobias in patients who have agoraphobia with panic attacks. The diagnostic criteria for panic disorder in DSM-III-R are as follows: The panic attacks (discrete periods of intense fear or discomfort), at least initially, are unexpected. Later in the course of this disturbance, certain situations (e.g. driving a car or being in a crowded place) may become associated with having a panic attack. These panic attacks are not triggered by situations in which the person is the focus of others' attention (as in social phobia). The diagnosis requires four such attacks within a four week period, or one or more attacks followed by at least a month of persistent fear of having another attack. The panic attacks must be characterised by at least four of the following symptoms: dyspnoea or smothering sensations; dizziness, unsteady feelings or faintness; palpitations or tachycardia; trembling or shaking; sweating; choking; nausea or abdominal distress; depersonalisation or derealisation; paraesthesiae; flushes (hot flashes) or chills; chest pain or discomfort; fear of dying; fear of going crazy or of doing something uncontrolled. Note. Attacks involving four or more symptoms are panic attacks; attacks involving fewer than four are limited symptom attacks. At least some of the panic attack symptoms must develop suddenly and increase in intensity within ten minutes of the beginning of the first symptom noticed in the attack. The panic attack must not be attributable to some known organic factor, e.g. amphetamine or caffeine, intoxication, hyperthyroidism. The efficacy of alprazolam in conditions where the above criteria are not met has not been established. The risk versus benefits of alprazolam use in milder disorders, which do not meet the above criteria, has not been evaluated. Although current evidence supports the long-term clinical effectiveness of alprazolam in panic disorder, the continuing use of alprazolam needs to be weighed against the difficulties that can occur with dependence and discontinuation. The results of a long-term study in patients taking alprazolam (ie. beyond three months) suggest that many patients continue to benefit from alprazolam therapy and that alprazolam efficacy is maintained for up to eight months. The physician should periodically reassess the usefulness of the drug for each patient. A comparative study of alprazolam and placebo in the treatment of panic attacks in patients with panic disorder involved 543 patients over an eight week period. Alprazolam was significantly more effective than placebo in reducing the total number of panic attacks (p < 0.0001); at week 4, 46.8% of alprazolam patients had achieved zero total panic attacks when compared to 27.1% of placebo patients. Panic disorders are often severe, chronic illnesses that cause a high level of work and social disability, increased substance abuse and potentially increased morbidity and mortality. Psychological and social factors are important in the pathogenesis of panic attacks, either acting alone or in combination with biological factors. Prolonged pharmacological therapy may be used as an adjunct to psychosocial therapy in the treatment of patients with panic disorders.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, uncoated
- Oral
- 9mm x 5.5mm oval, pale blue tablet debossed AL/1.0 on one side, G on the reverse.
Images


Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 25 degrees Celsius
- Shelf lifetime is 2 Years.
Do I need a prescription ?
(ARTG)
These medicines require authorisation for prescription from your doctor. It is Schedule 8 : Controlled Drug.
- 10 tablets_(1mg) pack
- 50 tablets pack
Is this medicine subsidised ?
(PBS)
This medicine was verified as being available on the PBS (Pharmaceutical Benefits Scheme) on October, 1 2025. To learn more about this subsidy, visit the Pharmaceutical Benefits Scheme (PBS) website.
Over 65 ?
(AHT)
This medicine contains the active ingredients:
If you are over 65 years of age, there may be specific risks and recommendations for use of this medicine. Please discuss your individual circumstances with your pharmacist, doctor or health professional. For more information read our page on medication safety for older people.
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient alprazolam
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: KALMA 0.5 alprazolam 0.5mg tablet blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
Listen to the audio transcription of the CMI leaflet.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
For short-term symptomatic treatment of anxiety including treatment of anxious patients with some symptoms of depression. Alprazolam is not recommended as primary therapy in patients with depression. For the treatment of panic disorder with or without some phobic avoidance, and for blocking or attenuation of panic attacks and phobias in patients who have agoraphobia with panic attacks. INDICATIONS AS AT 17 DECEMBER 2001 : Anxiety. Short-term symptomatic treatment of anxiety including treatment of anxious patients with some symptoms of depression. Panic disorder. The treatment of panic disorder with or without some phobic avoidance, and for blocking or attenuation of panic attacks and phobias in patients who have agoraphobia with panic attacks. The diagnostic criteria for panic disorder in DSM-III-R are as follows: The panic attacks (discrete periods of intense fear or discomfort), at least initially, are unexpected. Later in the course of this disturbance, certain situations (e.g. driving a car or being in a crowded place) may become associated with having a panic attack. These panic attacks are not triggered by situations in which the person is the focus of others' attention (as in social phobia). The diagnosis requires four such attacks within a four week period, or one or more attacks followed by at least a month of persistent fear of having another attack. The panic attacks must be characterised by at least four of the following symptoms: dyspnoea or smothering sensations; dizziness, unsteady feelings or faintness; palpitations or tachycardia; trembling or shaking; sweating; choking; nausea or abdominal distress; depersonalisation or derealisation; paraesthesiae; flushes (hot flashes) or chills; chest pain or discomfort; fear of dying; fear of going crazy or of doing something uncontrolled. Note. Attacks involving four or more symptoms are panic attacks; attacks involving fewer than four are limited symptom attacks. At least some of the panic attack symptoms must develop suddenly and increase in intensity within ten minutes of the beginning of the first symptom noticed in the attack. The panic attack must not be attributable to some known organic factor, e.g. amphetamine or caffeine, intoxication, hyperthyroidism. The efficacy of alprazolam in conditions where the above criteria are not met has not been established. The risk versus benefits of alprazolam use in milder disorders, which do not meet the above criteria, has not been evaluated. Although current evidence supports the long-term clinical effectiveness of alprazolam in panic disorder, the continuing use of alprazolam needs to be weighed against the difficulties that can occur with dependence and discontinuation. The results of a long-term study in patients taking alprazolam (ie. beyond three months) suggest that many patients continue to benefit from alprazolam therapy and that alprazolam efficacy is maintained for up to eight months. The physician should periodically reassess the usefulness of the drug for each patient. A comparative study of alprazolam and placebo in the treatment of panic attacks in patients with panic disorder involved 543 patients over an eight week period. Alprazolam was significantly more effective than placebo in reducing the total number of panic attacks (p < 0.0001); at week 4, 46.8% of alprazolam patients had achieved zero total panic attacks when compared to 27.1% of placebo patients. Panic disorders are often severe, chronic illnesses that cause a high level of work and social disability, increased substance abuse and potentially increased morbidity and mortality. Psychological and social factors are important in the pathogenesis of panic attacks, either acting alone or in combination with biological factors. Prolonged pharmacological therapy may be used as an adjunct to psychosocial therapy in the treatment of patients with panic disorders.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, uncoated
- Oral
- 9mm x 5.5mm oval, pale pink tablet debossed AL/0.5 on one side, G on the reverse.
Images


Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 25 degrees Celsius
- Shelf lifetime is 2 Years.
Do I need a prescription ?
(ARTG)
These medicines require authorisation for prescription from your doctor. It is Schedule 8 : Controlled Drug.
- 10 Tablets pack
- 50 tablets pack
Is this medicine subsidised ?
(PBS)
This medicine was verified as being available on the PBS (Pharmaceutical Benefits Scheme) on October, 1 2025. To learn more about this subsidy, visit the Pharmaceutical Benefits Scheme (PBS) website.
Over 65 ?
(AHT)
This medicine contains the active ingredients:
If you are over 65 years of age, there may be specific risks and recommendations for use of this medicine. Please discuss your individual circumstances with your pharmacist, doctor or health professional. For more information read our page on medication safety for older people.
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient alprazolam
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems









